Abstract
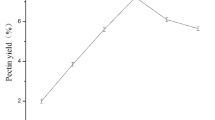
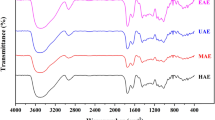
Jerusalem artichoke is an economic crop widely planted in saline-alkaline soil. The use of Jerusalem artichoke is of great significance. In this study, the response surface method was employed to optimize the effects of processing variables (extraction temperature, pH, extraction time, and liquid-to-solid ratio) on the yield of Jerusalem artichoke pectin. Under the optimal extraction conditions: pH 1.52, 63.62 min, 100°C and a liquid-to-solid ratio of 44.4 mL/g, the maximum pectin yield was predicted to be 18.76%. Experiments were conducted under these optimal conditions and a pectin yield of 18.52±0.90% was obtained, which validated the model prediction. The effects of diff erent drying methods (freeze drying, spray drying and vacuum drying) on the properties of Jerusalem artichoke pectin were evaluated and they were compared with apple pectin. FTIR spectral analysis showed no major structural diff erences in Jerusalem artichoke pectin samples produced by various drying treatments. The antioxidant activities of pectin dried by diff erent methods were investigated using in vitro hydroxyl and DPPH radical scavenging systems. The results revealed that the activities of spray dried pectin (SDP) and apple pectin (AP) were stronger than those of vacuum oven dried pectin (ODP) and vacuum freeze dried pectin (FDP). Therefore compared with the other two drying methods, the spray drying method was the best.
Similar content being viewed by others
References
Akashi K, Nishimura N, Ishida Y, Yokota A. 2004. Potent hydroxyl radical-scavenging activity of drought-induced type-2 metallothionein in wild watermelon. Biochemical and Biophysical Research Communications, 323 (1): 72–78.
Axelos M A V, Thibault J F, Lefebvre J. 1989. Structure of citrus pectins and viscometric study of their solution properties. International Journal of Biological Macromolecules, 11 (3): 186–191.
Bajpai P K, Bajpai P. 1991. Cultivation and utilization of Jerusalem artichoke for ethanol, single cell protein, and high-fructose syrup production. Enzyme and Microbial Technol ogy, 13 (4): 359–362.
Behall K, Reiser S. 1986. Effects of pectin on human metabolism. In: Fishman M L, Jen J J eds. Chemistry and Functions of Pectins (ACS Symposium), 310: 248–265.
Cacace J E, Mazza G. 2003. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J. Food Sci., 68 (1): 240–248.
Chen H X, Zhang M, Xie B J. 2004. Quantification of uronic acids in tea polysaccharide conjugates and their antioxidant properties. J. Agric. Food Chem., 52 (11): 3333–3336.
Gnanasambandam R, Proctor A. 1999. Determination of pectin degree of esterification by diff use reflectance Fourier transform infrared spectroscopy. Food Chemistry, 68 (3): 327–332.
Guo Z K, Jin Q H, Fan G Q, Duan Y P, Qin C, Wen M J. 2001. Microwave-assisted extraction of Effective constituents from a Chinese herbal medicine Radix puerariae. Analytica Chimica Acta, 43 6 (1): 41–47.
Guo Z Y, Xing R E, Liu S, Zhong Z M, Ji X, Wang L, Li P C. 2007. Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydr ate Res earch, 342 (10): 1329–1332.
Guo Z Y, Xing R E, Liu S, Yu H H, Wang P B, Li C P, Li P C. 2005. The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bioorg anic & Med icinal Chem istry Letters, 15 (20): 4600–4603.
Henrotin Y E, Bruckner P, Pujol J-P L. 2003. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis and Cartilage, 11 (10): 745–747.
Huang D J, Ou B X, Prior R L. 2005. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem., 53 (6): 1841–1856.
Iglesias M T, Lozano J E. 2004. Extraction and characterization of sunflower pectin. J ournal of Food Engineering, 62 (3): 215–223.
Kalapathy U, Proctor A. 2000. Effect of acid extraction and alcohol precipitation conditions on the yield and purity of soy hull pectin. Food Chemistry, 73 (4): 393–396.
Khasina E I, Kolenchenko E A, Sgrebneva M N et al. 2003. Antioxidant activities of a low etherified pectin from the seagrass Zostera marina. Russian J. Mar. Biol., 29 (4): 259–261.
Kishk Y F M, Al-Sayed H M A. 2007. Free-radical scavenging and antioxidative activities of some polysaccharides in emulsions. LWT-Food Science and Technology, 40 (2): 270–277.
Kulkarni S G, Vijayanand P. 2010. Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (Passiflora edulis f. flavicarpa L.). LWT-Food Science and Technology, 43 (7): 1026–1031.
Lü C, Wang Y, Wang L J, Li D, Adhikari B. 2013. Optimization of production yield and functional properties of pectin extracted from sugar beet pulp. Carbohydrate Polymers, 95 (1): 233–240.
Masmoudi M, Besbes S, Chaabouni M et al. 2008. Optimization of pectin extraction from lemon by-product with acidified date juice using response surface methodology. Carbohydrate Polymers, 74 (2): 185–192.
Mateos-Aparicio I, Mateos-Peinado C, Jiménez-Escrig A, Rupérez P. 2010. Multifunctional antioxidant activity of polysaccharide fractions from the soybean byproduct okara. Carbohydrate Polymers, 82 (2): 245–250.
May C D. 1990. Industrial pectins: sources, production and applications. Carbohydrate Polymers, 12 (1): 79–99.
Monsoor M A, Proctor A. 2001. Preparation and functional properties of soy hull pectin. J ournal of American Oil Chemist’s Society, 78 (7): 709–713.
Monsoor M A. 2005. Effect of drying methods on the functional properties of soy hull pectin. Carbohydrate Polymers, 61 (3): 362–367.
Pinheiro E R, Silva I M D A, Gonzaga L V, Amante E R, Teófilo R F, Ferreira M M C, Amboni D M C. 2008. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresource Technology, 99 (13): 5561–5566.
Rolin C. 1993. Pectin. In: BeMiller J N, Whistler R L eds. Industrial Gums: Polysaccharides and Their Derivatives. 3 rd edn. Elsevier Inc., New York, NY. p.257–293.
Singthong J, Cui S W, Ningsanond S, Goff H D. 2004. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydrate Polymers, 58 (4): 391–400.
Sun H H, Mao W J, Chen Y et al. 2009. Isolation, chemical characteristics and antioxidant properties of the polysaccharides from marine fungus Penicillium sp. F23-2. Carbohydrate Polymers, 78 (1): 117–124.
Tomida H, Yasufuku T, Fujii T, Kondo Y, Kai T, Anraku M. 2010. Polysaccharides as potential antioxidative compounds for extended-release matrix tablets. Carbohydr ate Res earch, 345 (1): 82–86.
Urias-Orona V, Huerta-Oros J, Carvajal-Millán E et al. 2010. Component analysis and free radicals Scavenging activity of Cicer arietinum L. Husk Pectin. Molecules, 15 (10): 6948–6955.
Vriesmann L C, Teófilo R F, Petkowicz C L O. 2011. Optimization of nitric acid-mediated extraction of pectin from cacao pod husks (Theobroma cacao L.) using response surface methodology. Carbohydrate Polymers, 84 (4): 1230–1236.
Wettasinghe M, Shahidi F. 1999. Evening primrose meal: a source of natural antioxidants and scavenger of hydrogen peroxide and oxygen-derived free radicals. J. Agric. Food Chem., 47 (5): 1801–1812.
Willats W G T, Knox J P, Mikkelsen J D. 2006. Pectin: new insights into an old polymer are starting to gel. Trends in Food Science & Technology, 17 (3): 97–104.
Xu W T, Zhang F F, Luo Y B et al. 2009. Antioxidant activity of a water-soluble polysaccharide purified from Pteridium aquilinum. Carbohydr ate Res earch, 344 (2): 217–222.
Yamada H. 1996. Contribution of pectins on health care. In: Visser J, Voragen A G J eds. Pectins and Pectinases. Progress in Biotechnology, Vol. 14. Elsevier. p.173–190.
Yamaguchi T, Takamura H, Matoba T, Terao J. 1998. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1, 1-diphenyl-2-picrylhydrazyl. Bioscience, Biotechnology, and Biochemistry, 62 (6): 1201–1204.
Yang S S, Cheng K T, Lin Y S, Liu Y W, Hou W C. 2004. Pectin hydroxamic acids exhibit antioxidant activities in vitro. J. Agric. Food Chem., 52 (13): 4270–4273.
Yapo B M, Wathelet B, Paquot M. 2007. Comparison of alcohol precipitation and membrane filtration Effects on sugar beet pulp pectin chemical features and surface properties. Food Hydrocolloids, 21 (2): 245–255.
Yuan W J, Zhao X Q, Ge X M, Bai F W. 2008. Ethanol fermentation with Kluyveromyces marxianus from Jerusalem artichoke grown in salina and irrigated with a mixture of seawater and freshwater. J ournal of Applied Microbiology, 105 (6): 2076–2083.
Zhang Z S, Wang F, Wang X M, Liu X L, Hou Y, Zhang Q B. 2010. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydrate Polymers, 82 (1): 118–121.
Zheng X Z, Yin F P, Liu C H, Xu X W. 2011. Effect of process parameters of microwave assisted extraction (MAE) on polysaccharides yield from Pumpkin. Journal of Northeast Agricultural University, 1 8 (2): 79–86.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National High Technology Research and Development Program of China (863 Program) (No. 2012AA021205) and the National Key Technology R&D Program of China (No. 2011BAC02B04)
Rights and permissions
About this article
Cite this article
Liu, S., Shi, X., Xu, L. et al. Optimization of pectin extraction and antioxidant activities from Jerusalem artichoke. Chin. J. Ocean. Limnol. 34, 372–381 (2016). https://doi.org/10.1007/s00343-015-4314-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-015-4314-4