Abstract
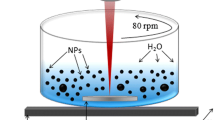
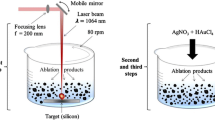
Hexagonally ordered mesoporous silica materials, MCM-41 and SBA-15, have been synthesized and loaded with Ag nanoparticles, utilizing both chemical synthesis and ultra-short pulsed laser ablation in liquid. In laser ablation, a silver target, immersed in aqueous suspension of ordered mesoporous silica SBA-15, was irradiated by ultra-short laser pulses to generate silver nanoparticles. For comparison, samples of similar silver contents were prepared either by incorporating silver into the SBA-15 during a hydrothermal synthesis or by introducing silver in MCM-41 by template ion-exchange. Samples were characterized by XRD, N2 physisorption, TEM and UV–vis spectroscopy. All preparations contained significant amount of 5–50 nm size silver agglomerates on the outer surface of the silica particles. The laser ablation process did not cause significant destruction of the SBA-15 structure and metallic silver (Ag0) nanoparticles were mainly generated. It is demonstrated that by laser ablation in aqueous silica suspension smaller and more uniform metallic silver particles can be produced and loaded on the surface of the silica support than by synthesis procedures. Catalytic properties of the samples have been tested in the total oxidation of toluene. Because of its favorable Ag dispersity, the Ag/SBA-15 catalyst, generated by the laser ablation method, had better catalytic stability and, relative to its Ag load, higher activity than the conventional Ag/SBA-15 preparations.






Similar content being viewed by others
References
A. De Bonis, A. Galasso, N. Ibris, A. Laurita, A. Santagata, R. Teghil, Appl. Surf. Sci. 268, 571–578 (2013)
A. De Giacomo, A. De Bonis, M. dell’Aglio, O. De Pascale, R. Gaudiuso, S. Orlando, A. Santagata, G.S. Senesi, F. Taccogna, R. Teghil, J. Phys. Chem. C 115, 5123–5130 (2011)
A. Santagata, A. De Bonis, A. De Giacomo, M. dell’Aglio, A. Laurita, G.S. Senesi, R. Gaudiuso, S. Orlando, R. Teghil, G.P. Parisi, J. Phys. Chem. C 115, 5160–5164 (2011)
K. Sasaki, N. Takada, Pure Appl. Chem. 82, 1317–1327 (2010)
F. Mafune, J. Kohno, Y. Takeda, T. Kondow, H. Sawabe, J. Phys. Chem. B 104, 8333–8337 (2000)
F. Mafune, J. Kohno, Y. Takeda, T. Kondow, H. Sawabe, J. Phys. Chem. B 104, 9111–9117 (2000)
F. Mafune, J. Kohno, Y. Takeda, T. Kondow, H. Sawabe, J. Phys. Chem. B 105, 5114–5120 (2001)
A. Henglein, J. Phys. Chem. 97, 5457–5471 (1993)
M.S. Yeh, Y.S. Yang, Y.P. Lee, H.F. Lee, Y.H. Yeh, C.S. Yeh, J. Phys. Chem. B 103, 6851–6857 (1999)
J. Neddersen, G. Chumanov, T.M. Cotton, Appl. Spectrosc. 47, 1959–1964 (1993)
M.S. Sibbald, G. Chumanov, T.M. Cotton, J. Phys. Chem. 100, 4672–4678 (1996)
A. De Giacomo, M. dell’Aglio, A. Santagata, R. Gaudiuso, O. De Pascale, P. Wagener, G.C. Messina, G. Compagnini, S. Barcikowski, Phys. Chem. Chem. Phys. 15, 3083–3092 (2013)
A. De Bonis, M. Sansone, L. D’Alessio, A. Galasso, A. Santagata, R. Teghil, J. Phys. D Appl. Phys. 46, 44530–44539 (2013)
V. Amendola, M. Meneghetti, Phys. Chem. Chem. Phys. 15, 3027–3046 (2013)
S. Barcikowski, G. Compagnini, Phys. Chem. Chem. Phys. 15, 3022–3026 (2013)
P. Wagener, S. Barcikowski, N. BårschPhotonik Int. 1, 20–23 (2011)
J. Zhu, Z. Konya, V.F. Puntes, I. Kiricsi, C.X. Miao, J.W. Ager, A.P. Alivisatos, G.A. Somorjai, Langmuir 19, 4396–4401 (2003)
Z. Kónya, V.F. Puntes, I. Kiricsi, J. Zhu, J.W. Ager, M.K. Ko, H. Frei, P. Alivisatos, G.A. Somorjai, Chem. Mater. 15, 1242–1248 (2003)
S. Rojas, F.J. Garcıa-Garcıa, S. Jaras, M.V. Martınez-Huerta, J.L. Fierro-Garcıa, M. Boutonnet, Appl. Catal. A Gen. 285, 24–35 (2005)
S. Bhattacharyya, A. Gabashvili, N. Perkas, A. Gedanken, J. Phys. Chem. C 111, 11161–11167 (2007)
S. Bhattacharyya, A. Gedanken, J. Phys. Chem. C 112, 659–665 (2008)
D.S. Gopala, R.R. Bhattacharjee, R. Richards, Appl. Organometal. Chem. 27, 1–5 (2013)
S. Hashimoto, T. Uwada, H. Masuhara, T. Asahi, J. Phys. Chem. C 112, 15089–15093 (2008)
G.-P. Yong, D. Tian, H.-W. Tong, S.-M. Liu, J. Mol. Catal. A Chem. 323, 40–44 (2010)
C.M. Maroneze, L.P. da Costa, F.A. Sigoli, Y. Gushikem, I.O. Mazali, Synth. Met. 160, 2099–2103 (2010)
Y. Chi, L. Zhao, Q. Yuan, Y. Li, J. Zhang, J. Tu, N. Li, X. Li, Chem. Eng. J. 195–196, 254–260 (2012)
S. Huh, J. Wiench, J. Chyoo, M. Pruski, V.S.Y. Lin, Chem. Mater. 15, 4247–4256 (2003)
W. Gac, A. Derylo-Marczewska, S. Pasieczna-Patkowska, N. Popivnyak, G. Zukocinski, J. Mol. Catal. A Chem. 268, 15–23 (2007)
W. Zhu, Y. Han, L. An, Microporous Mesoporous Mater. 80, 221–226 (2005)
D. Zhao, J. Feng, Q. Huo, N. Melosh, G.H. Fredrickson, B.F. Chmelka, G.D. Stucky, Science 279, 548–552 (1998)
A. Szegedi, Z. Kónya, D. Méhn, E. Solymár, G. Pál-Borbély, Z.E. Horváth, L.P. Biró, I. Kiricsi, Appl. Catal. A Gen. 272, 257–266 (2004)
U. Kreibig, M. Vollmer, Optical properties of metal clusters (Springer, New York, 1995)
N. Bogdanchikova, F.C. Meunier, M. Avalos-Borja, J.P. Breen, A. Pestryakov, Appl. Catal. B Environ. 36, 287–297 (2002)
X. Zhang, Z. Qu, F. Yu, Y. Wang, J. Catal. 297, 264–271 (2013)
Y. Sohn, J. Mol. Catal. A Chem. 379, 59–67 (2013)
L.F. Liotta, Appl. Catal. B Environ. 100, 403–412 (2010)
Acknowledgments
This paper draws on work undertaken as part of the project CLaN (Combined Laser Nanotechnology) co-financed by the Operational Programme ERDF Basilicata 2007–2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szegedi, Á., Popova, M., Valyon, J. et al. Comparison of silver nanoparticles confined in nanoporous silica prepared by chemical synthesis and by ultra-short pulsed laser ablation in liquid. Appl. Phys. A 117, 55–62 (2014). https://doi.org/10.1007/s00339-014-8499-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-014-8499-8