Abstract
Super-hydrophobic aluminum (Al) surfaces were successfully fabricated via electrochemical machining in neutral NaClO3 electrolyte and subsequent fluoroalkylsilane (FAS) modification. The effects of the processing time, processing current density, and electrolyte concentration on the wettability, morphology, and roughness were studied. The surface morphology, chemical composition, and wettability of the Al surfaces were investigated using scanning electron microscopy (SEM) equipped with energy-dispersive spectroscopy (EDS), white-light interferometry, roughness measurements, X-ray diffraction (XRD), Fourier-transform infrared spectrometry (FTIR), and optical contact angle measurements. The results show that hierarchical rough structures and low surface energy films were present on the Al surfaces after electrochemical machining and FAS modification. The combination of the rough structures and the low surface energy materials plays a crucial role in achieving super-hydrophobicity. Compared with the anodic oxidation and chemical etching method, the method proposed in our work does not require strong acid or alkali, and causes less harm to the environment and operators but with high processing efficiency. The rough structures required by the super-hydrophobic surfaces were obtained at 30-s processing time and the best super-hydrophobicity with 164.6∘ water contact angle and 2∘ tilting angle was obtained at 360 s. The resulting super-hydrophobic Al surfaces have a long-time stability in air and an excellent resistance to corrosive liquids.
















Similar content being viewed by others
References
W. Barthlott, C. Neinhuis, Planta 202, 1 (1997)
R. Furstner, W. Barthlott, C. Neinhuis, P. Walzel, Langmuir 21, 956 (2005)
P.N. Manoudis, I. Karapanagiotis, A. Tsakalof, I. Zuburtikudis, B. Kolinkeova, C. Panayiotou, Appl. Phys. A 97, 351 (2009)
M. Tang, M.H. Hong, Y.S. Choo, Z. Tang, D.H. Chua, Appl. Phys. A 101, 503 (2010)
T. Liu, Y.S. Yin, S.G. Chen, X.T. Chang, S. Cheng, Electrochim. Acta 52, 3709 (2007)
Y.S. Yin, T. Liu, S.G. Chen, T. Liu, T. Cheng, Appl. Surf. Sci. 255, 2978 (2008)
G. Mchale, N.J. Shirtcliffe, C.R. Evans, M.I. Newton, Appl. Phys. Lett. 94, 064104 (2009)
F. Shi, J. Niu, J.L. Liu, F. Liu, Z.Q. Wang, X.Q. Feng, X. Zhang, Adv. Mater. 19, 2257 (2007)
R. Jafari, M. Farzaneh, Appl. Phys. A 102, 195 (2011)
S.A. Kulinich, M. Farzaneh, Appl. Surf. Sci. 255, 8153 (2009)
L. Yin, Q. Xia, J. Xue, S.Q. Yang, Q.J. Wang, Q.M. Chen, Appl. Surf. Sci. 256, 6764 (2010)
L.L. Cao, A.K. Jones, V.K. Sikka, J.Z. Wu, D. Gao, Langmuir 25, 12444 (2009)
P. Tourkine, M.L. Merrer, D. Quere, Langmuir 25, 7214 (2009)
E. Stratakis, A. Mateescu, M. Barberoglou, M. Vamvakaki, C. Fotakis, S.H. Anastasiadis, Chem. Commun. 46, 4136 (2010)
M. Barberoglou, V. Zorba, A. Pagozidis, C. Fotakis, E. Stratakis, Langmuir 26, 13007 (2010)
K. Tsujii, T. Yamamoto, T. Onda, S. Shibuichi, Angew. Chem., Int. Ed. Engl. 26, 1011 (1997)
H. Wang, D. Dai, X.D. Wu, Appl. Surf. Sci. 254, 5599 (2008)
W.C. Wu, X.L. Wang, D.A. Wang, M. Chen, F. Zhou, W.M. Liu, Chem. Commun. 9, 1043 (2009)
Z.G. Guo, F. Zhou, J.C. Hao, W.M. Liu, J. Am. Chem. Soc. 127, 15670 (2005)
B.T. Qian, Z.Q. Shen, Langmuir 21, 9007 (2005)
Z.G. Guo, F. Zhou, J.C. Hao, W.M. Liu, J. Colloid Interface Sci. 303, 298 (2006)
M.N. Qu, B.W. Zhang, S.Y. Song, L. Chen, J.Y. Zhang, X.P. Cao, Adv. Funct. Mater. 17, 593 (2007)
D.K. Sarkar, M. Farzaneh, R.W. Paynter, Mater. Lett. 62, 1226 (2008)
J.L. Song, W.J. Xu, Y. Lu, J. Mater. Sci. 1, 162 (2011)
R.N. Wenzel, Ind. Eng. Chem. 28, 988 (1936)
Y.F. Li, Z.J. Yu, S.B. Huo, S.P. Song, J. Chem. Eng. Chin. Univ. 22, 6 (2008)
A.R. Eivani, H. Ahmed, J. Zhou, J. Duszczyk, Metall. Mater. Trans. A, Phys. Metall. Mater. Sci. 40, 717 (2009)
W.M. Mao, L. Chen, L.M. Sa, Y.N. Yu, Y.F. Li, Chin. J. Nonferr. Met. 14, 1 (2004)
T. Ishizaki, Y. Masuda, M. Sakamoto, Langmuir 27, 4780 (2011)
A.B.D. Cassie, S. Baxter, Trans. Faraday Soc. 40, 546 (1944)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC, Grant No. 90923022).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
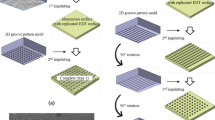
As is known, the self-assembly of alkylsilane is one of the efficient strategies to introduce low surface energy compounds on a surface to enhance surface hydrophobicity. Moreover, the alkylsilane is promising for practical applications because of its marked mechanical and chemical stability due to the strong immobilization through siloxane bondings. So, here, the fluoroalkylsilane (FAS), which contains the –CF3 group with a surface energy of 6.7 mJ/m2 and the –CF2− group with a surface energy of 18 mJ/m2, was used to decrease the free energy of the Al surfaces. The self-assembly process of FAS on the Al surfaces is shown in Fig. 17. First, the silicon ethoxide (Si–OC2H5) functional groups react with water to form silanols (Si–OH), which act as the reactive groups at the end of the molecule. The silanols subsequently react with the –OH groups to form a self-assembled film. Meanwhile, the surface can also induce vertical polymerization to form a grafted polysiloxane.
The surface chemical compositions of the super-hydrophobic Al surfaces obtained by the electrochemical machining and FAS modification were investigated by FTIR and EDS. Figure 18 shows the FTIR spectrum of the super-hydrophobic Al surfaces. Five absorption bands at around 1244, 1214, 1167, 1146, and 1122 cm−1 are assigned to the C–F stretching vibration of –CF2– and –CF3 groups of the FAS molecules. The band at 1076 cm−1 is identified as the framework vibration of Si–O–Si bonds. The bands at less than 897 cm−1 are due to the stretching vibration of Si–C bonds. All this indicates that the super-hydrophobic Al surfaces have been uniformly covered by the FAS film. Figure 19 shows the EDS spectra of the Al surfaces before and after FAS modification. It can be seen that the Al surfaces after electrochemical machining and before FAS modification just contain the element Al, while the super-hydrophobic Al surfaces mainly consist of elemental Al, C, O, F, and Si, indicating that the FAS film has self-assembled on the Al surfaces.
Rights and permissions
About this article
Cite this article
Song, Jl., Xu, Wj., Liu, X. et al. Electrochemical machining of super-hydrophobic Al surfaces and effect of processing parameters on wettability. Appl. Phys. A 108, 559–568 (2012). https://doi.org/10.1007/s00339-012-6927-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-012-6927-1