Abstract
Objectives
The magnetic resonance (MR) 4D flow imaging–derived left atrial (LA) acceleration factor α was recently introduced as a means to non-invasively estimate LA pressure. We aimed to investigate the association of α with the severity of left ventricular (LV) diastolic dysfunction using echocardiography as the reference method.
Methods
Echocardiographic assessment of LV diastolic function and 3-T cardiac MR 4D flow imaging were prospectively performed in 94 subjects (44 male/50 female; mean age, 62 ± 12 years). LA early diastolic peak outflow velocity (vE), systolic peak inflow velocity (vS), and early diastolic peak inflow velocity (vD) were evaluated from 4D flow data. α was calculated from α = vE / [(vS + vD) / 2]. Mean parameter values were compared by t-test; diagnostic performance of α in predicting diastolic (dys)function was investigated by receiver operating characteristic curve analysis.
Results
Mean α values were 1.17 ± 0.14, 1.20 ± 0.08, 1.33 ± 0.15, 1.77 ± 0.18, and 2.79 ± 0.69 for grade 0 (n = 51), indeterminate (n = 9), grade I (n = 13), grade II (n = 13), and grade III (n = 8) LV diastolic (dys)function, respectively. α differed between subjects with non-advanced (grade < II) and advanced (grade ≥ II) diastolic dysfunction (1.20 ± 0.15 vs. 2.16 ± 0.66, p < 0.001). The area under the curve (AUC) for detection of advanced diastolic dysfunction was 0.998 (95% CI: 0.958–1.000), yielding sensitivity of 100% (95% CI: 84–100%) and specificity of 99% (95% CI: 93–100%) at cut-off α ≥ 1.58. The AUC for differentiating grade III diastolic dysfunction was also 0.998 (95% CI: 0.976–1.000) at cut-off α ≥ 2.14.
Conclusion
The 4D flow–derived LA acceleration factor α allows grade II and grade III diastolic dysfunction to be distinguished from non-advanced grades as well as from each other.
Clinical relevance statement
As a single continuous parameter, the 4D flow–derived LA acceleration factor α shows potential to simplify the multi-parametric imaging algorithm for diagnosis of advanced LV diastolic dysfunction, thereby identifying patients at increased risk for cardiovascular events.
Key Points
• Detection of advanced diastolic dysfunction is typically performed using a complex, multi-parametric approach.
• The 4D flow–derived left atrial acceleration factor α alone allows accurate detection of advanced left ventricular diastolic dysfunction.
• As a single continuous parameter, the left atrial acceleration factor α could simplify the diagnosis of advanced diastolic dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Differentiation between left ventricular (LV) diastolic dysfunction with normal LV filling pressure from LV diastolic dysfunction with elevated LV filling pressure plays an important role in treatment and management of patients with heart failure. The LV filling pressure is considered elevated when the mean left atrial (LA) pressure, or its surrogate, the mean pulmonary arterial wedge pressure (PAWP), exceeds 15 mmHg [1]. Echocardiography represents the established non-invasive reference method for the evaluation and grading of LV diastolic dysfunction from a multi-parametric, threshold-based algorithm [2]. Cut-off values for the transmitral and mitral annular diastolic peak velocities and peak velocity ratios are employed together with cut-off values for the maximal tricuspid regurgitation velocity and the maximal LA volume to differentiate between normal LV diastolic function (grade 0 diastolic dysfunction), indeterminate diastolic function, and grades I–III diastolic dysfunction. Advanced LV diastolic dysfunction, defined as diastolic dysfunction of grade II or III, is considered to be associated with increased LV filling pressures [2]. The accurate detection of advanced diastolic dysfunction by echocardiography, however, can be challenging [3, 4].
The LA acceleration factor α derived from magnetic resonance (MR) 4D flow imaging was recently identified as a non-invasive means of estimating PAWP in subjects with pulmonary hypertension or at risk of having pulmonary hypertension [5]. Defined as the ratio of the 4D flow–derived early diastolic LA peak outflow velocity to the average of systolic and early diastolic LA peak inflow velocities, α correlated very highly with PAWP assessed by right heart catheterization and allowed the prediction of PAWP > 15 mmHg with high sensitivity and specificity.
As advanced LV diastolic dysfunction is expected to exhibit increased filling pressures [2], we hypothesized that α—as a single parameter—would allow discrimination between advanced and non-advanced forms of diastolic dysfunction. The aim of the present proof-of-principle study was therefore to investigate the relationship between α and the grades of LV diastolic dysfunction as evaluated from echocardiography.
Materials and methods
Study population
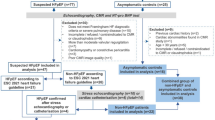
Between October 2016 and February 2022, 96 adult subjects (age > 18 years) were prospectively recruited for echocardiography and MR 4D flow imaging. The study cohort consisted of 61 subjects without signs or symptoms of cardiovascular disease and without known cardiac disease (ClinicalTrials.gov, NCT01728597) and 35 consecutive subjects with referral for cardiac MR with known or suspected diastolic dysfunction documented in their medical records (ClinicalTrials.gov, NCT03253835). The study was approved by the local ethics review board and complied with the Declaration of Helsinki. Written informed consent was obtained from all participants. Exclusion criteria were contraindications for MR imaging, known pregnancy or claustrophobia, irregular heart rhythm, significant mitral stenosis, or implanted cardiac devices. Demographic (age, gender) and available clinical data (medical history, blood pressure, blood laboratory data) were collected at the time of inclusion. Two subjects did not complete cardiac MR imaging because of claustrophobia (n = 1) and severe back pain during the investigation (n = 1). Therefore, 94 patients were included in the analysis. Figure 1 presents the subject flowchart of the study.
Study flowchart. Diagnosis for the patients undergoing the contrast enhanced examinations: IHD, ischemic heart disease; AMY, cardiac amyloidosis; HCM, hypertrophic cardiomyopathy; AST, aortic stenosis; HHD, hypertensive heart disease; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; DCM, dilatative cardiomyopathy; RCM, restrictive cardiomyopathy
Transthoracic echocardiography and grading of diastolic (dys)function
Echocardiographic examinations were performed according to the 2016 American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) recommendations [2], using a Vivid E9 system (GE HealthCare). Briefly, images were acquired with simultaneous electrocardiographic recording during quiet respiration and with the patient in a left lateral decubitus position. The LV ejection fraction (EF) and maximal LA volume indexed to the body surface area (LAVI) were evaluated from apical 4-chamber and 2-chamber view series using the biplane Simpson method and the biplanar area-length method, respectively. Early diastolic and late diastolic transmitral peak velocities (referred to hereafter as E and A, respectively) were measured in the apical 4-chamber view. Early diastolic tissue Doppler mitral annular peak velocity eʹ was calculated as the average of the septal and lateral velocities acquired in the apical 4-chamber view. The evaluation of the peak tricuspid regurgitation velocity (TR) was performed with continuous-wave Doppler in the apical 4-chamber view. Additionally, systolic and early diastolic pulmonary venous peak velocities (referred to hereafter as S and D, respectively) were measured in the right upper or right lower pulmonary vein, visualized in the apical 4-chamber view. After optimization of the transducer position, the Doppler sample volume was placed about 0.5 cm into the pulmonary vein. Moreover, the systolic pulmonary arterial pressure (sPAP) was estimated from TR (in m/s) via sPAP (in mmHg) = 4∙TR2 + 5 mmHg [6].
All echocardiographic parameters were evaluated from series of three to five consecutive cardiac cycles. Diastolic function was assessed employing the ASE/EACVI proposed algorithm [2]: In subjects with normal LV EF (≥ 50%), cut-offs for eʹ, E/eʹ, peak tricuspid regurgitation velocity, and LAVI were used to grade LV diastolic function as normal (grade 0, < 50% of echocardiographic cut-offs positive), indeterminate (50% positive), or diastolic dysfunction (> 50% positive). In subjects with diastolic dysfunction, as well as subjects with reduced LV EF or with a structural heart disease, grade of diastolic dysfunction (I–III) was determined using above parameter together with E and E/A.
4D flow imaging
MR 4D flow imaging was performed on a 3-T scanner (Magnetom Skyra, Siemens Healthineers) using a phased-array 18-channel body matrix together with a spine matrix coil. All subjects were investigated under shallow breathing in the supine position. Multislice 4D flow data were acquired from stacks of retrospectively electrocardiographically or pulse-gated 2-dimensional phase-contrast series with three-directional velocity encoding [7, 8], covering at least the LV and LA in 3-chamber-view orientation. In subjects referred for cardiac MR (n = 34), 4D flow imaging was performed 15–20 min after application of 0.2 mmol/kg gadobutrol (Gadovist; Bayer Schering Pharma). Parameters of the 4D flow protocol were as follows: voxel size, 1.8 × 2.5 × 4 mm3; measured temporal resolution, 41.8 ms interpolated to 30 cardiac phases per cardiac cycle; echo time, 3.1 ms; flip angle, 12° (native) or 15° (contrast enhanced); parallel acquisition factor, 2; velocity encoding, 100–190 cm/s in all directions (adjusted to prevent aliasing in LV and LA); number of averages, 2; typical acquisition time, 45 s per slice (50 heart beats) or 22 min to cover the heart (20–40 gapless slices).
4D flow data analysis
Pre-processing (background phase correction, aliasing correction if necessary) and post-processing of 4D flow data were performed using prototype software (4Dflow, Siemens Healthineers). Early diastolic peak LA outflow velocity (vE) was assessed from the voxel with the highest velocity in an evaluation plane reconstructed parallel to the mitral valve at the level of the mitral valve annulus in early diastole. For the evaluation of the systolic (vS) and early diastolic (vD) peak LA inflow velocities, the cross sections of the left and right lower and upper pulmonary veins were reconstructed at the levels of the atrial junctions in systole and early diastole, respectively (Fig. 2). The typical duration for the total 4D flow analysis was 10 min per case. The evaluation was performed by a reader with 7 years of experience (C.R.). To investigate interobserver reliability of the derived variables, the evaluation was repeated for 20 randomly selected subjects by a second reader with 20 years of experience (U.R.), who was blinded to the previous results.
Evaluation of early diastolic LA peak outflow velocity (vE) at the atrioventricular junction (left) and LA peak inflow velocities during systole (vS) (middle) and early diastole (vD) (right) at the orifice of the pulmonary vein into the left atrium from 4D flow data. White dotted lines indicate the multiplanar reconstructed evaluation planes for peak velocities. Plus signs (lower panel) indicate the locations of the voxels with the peak velocities employed for calculation of the LA acceleration factor
LA acceleration factor
Following the definition of the LA acceleration factor α as the ratio of vE to the average of vS and vD [5], α was calculated from 4D flow data according to the formula α = vE / [(vS + vD) / 2], where the maximum of vS + vD among all pulmonary veins was employed. Additionally, the LA acceleration factors αlower, derived by the same formula but using the maximum vS + vD among the lower pulmonary veins (as only these were evaluated in Reiter et al [5]), and αecho = E / [(S + D) / 2], derived from corresponding echocardiographic Doppler measurements, were calculated for comparative purposes.
Statistical analysis
Mean values are given together with standard deviations; areas under the curve (AUC), sensitivities, and specificities are specified together with 95% confidence intervals in parentheses. Statistical analysis was performed using SPSS® v28 (IBM). For statistical tests, a significance level of 0.05 was employed.
Comparisons between corresponding parameters derived from MR and echocardiographic investigations or measurements employing all or only the lower pulmonary veins were performed by paired t-test after assessing normality by the Shapiro-Wilk test. Means of different groups of diastolic (dys)function were compared by analysis of variance with Dunett-T3 as a post hoc test; α for reduced and normal EF was compared by the Mann-Whitney U test. The relationships of continuous parameters were investigated by means of correlation, linear regression, and Bland-Altman analysis. Correlations are termed according to the correlation coefficients as negligible (0.0–0.3), low (0.3–0.5), moderate (0.5–0.7), high (0.7–0.9), or very high (0.9–1.0) [9]. Diagnostic performance of LA acceleration factors in discriminating advanced diastolic dysfunction was assessed with receiver operating characteristic (ROC) curve analysis, and 95% confidence intervals were calculated for area under the ROC curve (AUC), sensitivity, and specificity; non-overlapping confidence intervals were interpreted as different. Reported cut-off values correspond to the maximum of Youden’s index.
Interobserver reliabilities for determination of peak velocities and acceleration factors were determined by within-subject standard deviation (SDw) in variance component analysis and two-way mixed effect model, single measure, absolute agreement intra-class correlation coefficients (ICC). Interobserver reliability is termed poor (ICC 0.0–0.5), moderate (ICC 0.5–0.75), good (ICC 0.75–0.9), and excellent (0.9–1.0) [10].
Results
Study population
The analyzed study population consisted of 94 subjects (females/males, 50/44; age, 62 ± 12 years) who underwent echocardiography and cardiac MR imaging within 3 ± 7 days. The mean heart rates during echocardiography (68 ± 12 min−1) and 4D flow imaging (67 ± 11 min−1) did not differ (p = 0.334). Echocardiography diagnosed normal LV diastolic function in 51 subjects (54%), indeterminate LV diastolic (dys)function in 9 subjects (10%), and grade I diastolic dysfunction in 13 subjects (14%). Advanced LV diastolic dysfunction was diagnosed in 21 subjects (22%), with 13 subjects (14%) classified as having grade II and 8 subjects (9%) classified as having grade III diastolic dysfunction. The demographic characteristics of the study population are summarized in Table 1 together with echocardiographic and blood laboratory parameters. No subject had significant mitral or aortic regurgitation; 10 subjects demonstrated reduced EF.
LA acceleration factor α
Means of peak LA inflow and outflow velocities as well as corresponding LA acceleration factors for the total study population and for the groups of patients with diastolic dysfunction are summarized in Table 2. In 83 subjects (88%), the highest average LA inflow velocities were measured in the upper left (56%) or upper right (32%) pulmonary vein; the highest average LA inflow velocities were observed in the left lower pulmonary vein in 8 subjects (9%) and in the right lower pulmonary vein in 3 subjects (3%). α correlated significantly with all continuous echocardiographic parameters used for grading: EF (r = −0.27, p = 0.01), E (r = 0.40, p < 0.001), E/A (r = 0.60, p < 0.001), eʹ (r = −0.37, p = 0.009), E/eʹ (r = 0.66, p < 0.001), LAVI (r = 0.43, p < 0.001), TR (0.42, p < 0.001), and sPAP (r = −0.494, p < 0.001). α differed between subjects with normal and reduced EF (1.35 ± 0.46 vs. 1.99 ± 0.70, p < 0.001). Moreover, there were significant correlations of α with laboratory parameters Hb (r = −0.25, p = 0.02), eGFR (r = −0.49, p < 0.001), NTproBNP (r = 0.53, p < 0.001), and LDL (r = −0.34, p < 0.001). Maximum vE, vS, (vS + vD) / 2, and α differed among the groups of patients with diastolic (dys)function, whereby means of vS and (vS + vD) / 2 decreased while α increased with grade of diastolic dysfunction. Notably, means of α for diastolic dysfunction grade II and grade III differed from those of all other groups (Fig. 3).
Compared to normal, indeterminate, and grade I diastolic dysfunction, α was higher in advanced diastolic dysfunction (1.20 ± 0.15 vs. 2.16 ± 0.66, p < 0.001). The AUC for the detection of advanced diastolic dysfunction from α was 0.998 (0.958–1.000) (Fig. 4). With the cut-off value α ≥ 1.58, sensitivity and specificity for detection of advanced diastolic dysfunction were 100% (84–100%) and 99% (93–100%), respectively. The AUC for differentiation of grade III diastolic dysfunction was 0.998 (0.976–1.000). Using a cut-off value α ≥ 2.14, sensitivity and specificity for detection of grade III diastolic dysfunction were 100% (63–100%) and 99% (94–100%), respectively.
Within the subjects with non-advanced diastolic dysfunction, the AUC for detection of normal diastolic function from α was 0.711 (0.569–0.812) (sensitivity and specificity of 71% (56–83%) and 68% (45–86%) at a cut-off value α ≤ 1.22) and 0.797 (0.646–0.888) for detection of grade I diastolic dysfunction (sensitivity and specificity of 77% (46–95%) and 72% (59–83%) at a cut-off value α ≥ 1.24).
LA acceleration factor from lower pulmonary veins α lower
Means of maximal (vS + vD) / 2 were smaller when evaluated from the lower pulmonary veins, both in total and for the groups of diastolic (dys)function (p = < 0.001–0.045) (Table 2). Consequently, a significant bias of −0.27 (p < 0.001) between α and αlower was observed. The correlation between α and αlower was, however, very high (r = 0.90, p < 0.001) (Fig. 5).
Bland-Altman plot (a) and scatter plot with linear regression analysis (b) of the LA acceleration factors derived from all pulmonary veins (α) and from only the lower pulmonary veins (αlower). Gray bar indicates the 95% confidence interval of the bias in the case of the Bland-Altman plot and the 95% confidence limits of the regression line in the case of the scatter plot. LoA, limit of agreement; r, correlation coefficient
The AUC for the detection of advanced diastolic dysfunction from αlower was 0.961 (0.899–0.990) and did not differ from the corresponding AUC derived from α. With the cut-off value αlower ≥ 1.77, sensitivity and specificity for the prediction of advanced diastolic dysfunction were 90% (70–99%) and 89% (80–95%), respectively.
Interobserver reliability
While the interobserver reliability for the determination of peak velocities ranged from good to excellent, the interobserver reliability of α as well as αlower was excellent (Table 3).
LA acceleration factor from echocardiographic measurements α echo
Means of E, S, D, and (S + D) / 2 as well as corresponding LA acceleration factor αecho for the total study population and for the groups of patients with diastolic dysfunction are summarized in Table 4. Due to inadequate visualization of the pulmonary veins in the apical 4-chamber view, S and D measurements could not be performed in 5 subjects; among the remaining 89 subjects, the right lower pulmonary vein was evaluated in 81 subjects (91%) and the right upper pulmonary vein in 8 subjects (9%). While low correlations were observed between vE and E (r = 0.49, p < 0.001), the correlation between maximal (vS + vD) / 2 and (S + D) / 2 was negligible (r = 0.25, p = 0.017).
α and αecho showed a significant bias (−0.25, p < 0.001), large standard deviation of differences (SD = 0.53), and a low correlation (r = 0.48, p < 0.001) (Fig. 6). The AUC for the detection of advanced diastolic dysfunction from αecho was 0.692 (0.535–0.848).
Bland-Altman plot (a) and scatter plot with linear regression analysis (b) of the LA acceleration factors derived from 4D flow (α) and from echocardiography (αecho). Gray bar indicates the 95% confidence interval of the bias in the case of Bland-Altman plot and the 95% confidence limits of the regression line in the case of the scatter plot. LoA, limit of agreement; r, correlation coefficient
Discussion
Our study yielded three main findings: The 4D flow–derived LA acceleration factor α (1) allows differentiation of grade II and III diastolic dysfunction from normal diastolic function as well as from non-advanced grades of diastolic dysfunction, (2) shows a very high correlation but a bias to the LA acceleration factor αlower determined from the lower pulmonary veins, and (3) is only weakly correlated with αecho derived from echocardiographic measurements.
The 4D flow–derived LA acceleration factor α was initially introduced as a non-invasive means of estimating PAWP in patients at risk for or with pulmonary hypertension [5]; the present study confirms the association between α and PAWP in a heterogeneous cohort of patients by showing that α allows differentiation between normal, indeterminate, or grade I diastolic dysfunction and advanced diastolic dysfunction, in which LV filling pressure can be assumed to be increased. α showed significant but only low to moderate correlations with echocardiographic E, E/A, E/eʹ, and LAVI. Comparable correlations have previously been found between echocardiography and invasively measured PAWP [11, 12], further supporting an association between α and PAWP in the current study population, which included subjects with normal LV EF as well as patients with hypertrophic cardiomyopathy, restrictive cardiomyopathy, or aortic stenosis.
Evidence of advanced diastolic dysfunction—or increased LV filling pressures—is not only an important aspect in the diagnosis of heart failure (especially when LV EF is preserved), but also identifies patients at increased risk for cardiovascular events and mortality in a wide variety of conditions [13,14,15], e.g., in patients on hemodialysis [16, 17], pre-dialysis chronic kidney disease [18], in patients with reduced and normal LV EF [19, 20], patients with ischemic heart failure [21], after cardiac surgery [22], or after transcatheter aortic valve replacement [23, 24].
The established method for non-invasive detection of elevated LV filling pressures is a complete grading of LV diastolic dysfunction using a complex multi-parametric echocardiographic algorithm with thresholds for E, E/A, E/eʹ, eʹ, TR, and LAVI [2]; cardiac MR imaging–based assessment mimicking the echocardiographic diagnostic flowchart is possible [6, 25, 26] but currently not established. The MR 4D flow–derived parameter α provides a conceptually simple and reliable single-parameter solution for this task, which other known parameters (from echocardiography or from MR) cannot fulfill with comparable accuracy: The diagnostic accuracy of the most commonly used diastolic parameter E/eʹ is limited in subjects with normal LV EF [27, 28] as well as in patients with pulmonary hypertension [29, 30], patients with hypertrophic cardiomyopathy [31], and in acutely decompensated patients with reduced EF [32]. The association between enlarged LAVI and elevated LV filling pressure is impaired in—among others—athletes, patients with mitral valve disease, patients with atrial fibrillation [2, 33], and patients with persistent LA dilatation after heart failure therapy [34]. While reduced LA strains have been shown to better predict elevated LV filling pressures compared with other single diastolic parameters [33, 35,36,37], the diagnostic accuracy of LA strains is nevertheless inferior to that of α.
In the model proposed in [5] for non-invasive estimation of PAWP, αlower was calculated from the maximum pulmonary venous inflow velocities of the lower left or right pulmonary veins. In the present study, α was derived from the maximum pulmonary venous inflow velocities among all pulmonary veins. Interestingly, in the majority of subjects, the highest (vS + vD) / 2 value was measured in the upper left or right pulmonary vein. Due to the very high correlation between α and αlower, no significant difference was observed for the discrimination of advanced diastolic dysfunction from α and αlower, suggesting that the diagnosis of advanced diastolic dysfunction could in principle be determined in a shorter examination time from a 4D flow stack that does not cover the entire LA volume. It should, however, be noted that due to the observed bias between α and αlower, calculation of PAWP from the formula PAWP = −6.2 + 10.1·αlower given in [5] underestimates PAWP by approximately 3 mmHg when used for α (e.g., a cut-off of α = 1.58 for advanced diastolic dysfunction translates to a PAWP of 13 rather than 10 mmHg, matching well with pressures observed by Andersen et al [12] for different grades of diastolic dysfunction).
The small but significant higher α in the grade I diastolic dysfunction group compared with the normal diastolic function group and the significant AUCs for differentiation between normal and grade I diastolic (dys)function within the non-advanced diastolic dysfunction group might indicate a slightly higher PAWP in grade I diastolic dysfunction that is still within in the normal range. The α difference of 0.14 between normal and grade I diastolic (dys)function would translate into a calculated PAWP difference of approximately 1.5 mmHg, which was similarly invasively determined in [38].
The echocardiographic analog αecho of α, derived via the recommended procedure to measure peak pulmonary venous velocities S and D as well as early diastolic transmitral peak velocity E, neither correlated well with α nor allowed accurate detection of advanced diastolic dysfunction. Apart from the facts that (S + D) / 2 was only derived from one pulmonary vein and that pulmonary venous flow is not always assessable with optimal quality by transthoracic echocardiography [39, 40], these findings can be attributed largely to the difference in anatomic localization of LA inflow and outflow velocity measurements on echocardiography as opposed to MR imaging. As shown by measurements of peak velocities within 4D flow datasets, correlation of PAWP and LA acceleration factor dramatically decreases if velocities are not derived at the atrioventricular junction and the orifice of the pulmonary veins but at the mitral valve tips and 1 cm into the pulmonary vein [5].
The study did not aim to optimize time of 4D flow acquisition or the analysis time. The multislice 4D flow protocol was chosen to optimize the “anatomical” contrast in native 4D flow acquisitions [41]. Apart from the aforementioned reduction of the stack volume, acquisition time could be further reduced by using effective navigator gating and/or compressed sensing techniques [42, 43]. This could allow acquisition of 4D flow after contrast application and before late-enhancement imaging. The 4D flow evaluation time included the measurement of systolic and early diastolic peak velocities in all pulmonary veins as well as their anatomical categorization. Determination of the fastest flow or at least two candidates for the fastest flow can be typically performed visually, and their categorization is not required for calculation of α. Thus, even without any automation, a potential halving of the evaluation time would be achievable.
We acknowledge the following limitations of our study: It was a single-center proof-of-principle study that investigated the potential of α to predict advanced LV diastolic dysfunction. Invasive hemodynamic measurements were not available for the study cohort. The time difference between the cardiac MR imaging study and the echocardiography was kept short but changes in filling pressures may have occurred. Moreover, patients with irregular heart rhythm, patients with significant mitral stenosis, or patients with congenital heart disease in whom echocardiographic assessment of LV diastolic function is limited [2] were not included in the study. Therefore, the results are not applicable to such subjects. Finally, it should be mentioned that α was evaluated from native and post-contrast 4D flow datasets. However, the application of contrast agent should not directly affect velocities measured by MR phase-contrast imaging [44].
In conclusion, the 4D flow–derived LA acceleration factor α, on its own, allows grade II and grade III diastolic dysfunction to be distinguished from non-advanced grades as well as from each other. Reducing the 4D flow dataset to cover the lower pulmonary veins when calculating α is associated with a bias of this parameter but appears to cause only a non-significant decrease in predictive accuracy. The LA acceleration factor derived from standard transmitral and pulmonary venous echocardiographic measurements, on the other hand, cannot be used to distinguish advanced diastolic dysfunction.
Abbreviations
- 4D flow:
-
Four-dimensional (time-resolved, three-directional) phase-contrast imaging
- α :
-
Left atrial acceleration factor
- α echo :
-
Left atrial acceleration factor assessed from echocardiography
- α lower :
-
Left atrial acceleration factor assessed from lower pulmonary veins
- A :
-
Late diastolic transmitral peak velocity
- AUC:
-
Area under the receiver operating characteristic curve
- D :
-
Early diastolic pulmonary venous peak velocity
- E :
-
Early diastolic transmitral peak velocity
- eʹ:
-
Early diastolic mitral valve tissue peak velocity
- EF:
-
Ejection fraction
- ICC:
-
Intra-class correlation coefficients
- LA:
-
Left atrium
- LAVI:
-
Left atrial volume index
- LDL:
-
Low-density lipoprotein
- LV:
-
Left ventricle
- MR:
-
Magnetic resonance
- NTproBNP:
-
N-terminal prohormone of brain natriuretic peptide
- PAWP:
-
Mean pulmonary arterial wedge pressure
- ROC:
-
Receiver operating characteristic curve
- S :
-
Systolic pulmonary venous peak velocity
- SDw :
-
Within-subject standard deviation
- sPAP:
-
Systolic pulmonary arterial pressure
- TR:
-
Peak tricuspid regurgitation velocity
- v D :
-
Early diastolic left atrial peak inflow velocity
- v E :
-
Early diastolic left atrial peak outflow velocity
- v S :
-
Systolic left atrial peak inflow velocity
References
McDonagh TA, Metra M, Adamo M et al (2021) 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42:3599–3726. https://doi.org/10.1093/eurheartj/ehab368
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29:277–314. https://doi.org/10.1016/j.echo.2016.01.011
Nauta JF, Hummel YM, van der Meer P et al (2018) Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 20:1303–1311. https://doi.org/10.1002/ejhf.1220
Abudiab MM, Chebrolu LH, Schutt RC et al (2017) Doppler echocardiography for the estimation of LV filling pressure in patients with mitral annular calcification. JACC Cardiovasc Imaging 10:1411–1420. https://doi.org/10.1016/j.jcmg.2016.10.017
Reiter G, Kovacs G, Reiter C et al (2022) Left atrial acceleration factor as a magnetic resonance 4D flow measure of mean pulmonary artery wedge pressure in pulmonary hypertension. Front Cardiovasc Med 9:972142. https://doi.org/10.3389/fcvm.2022.972142
Ramos JG, Fyrdahl A, Wieslander B et al (2020) Cardiovascular magnetic resonance 4D flow analysis has a higher diagnostic yield than Doppler echocardiography for detecting increased pulmonary artery pressure. BMC Med Imaging 20:28. https://doi.org/10.1186/s12880-020-00428-9
Reiter U, Kovacs G, Reiter C et al (2020) MR 4D flow-based mean pulmonary arterial pressure tracking in pulmonary hypertension. Eur Radiol. https://doi.org/10.1007/s00330-020-07287-6
Pelc NJ, Bernstein MA, Shimakawa A, Glover GH (1991) Encoding strategies for three-direction phase-contrast MR imaging of flow. J Magn Reson Imaging 1:405–413. https://doi.org/10.1002/jmri.1880010404
Mukaka MM (2012) Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J J Med Assoc Malawi 24:69–71
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Venkateshvaran A, Tureli HO, Faxén UL et al (2022) Left atrial reservoir strain improves diagnostic accuracy of the 2016 ASE/EACVI diastolic algorithm in patients with preserved left ventricular ejection fraction: insights from the KARUM haemodynamic database. Eur Heart J Cardiovasc Imaging 23:1157–1168. https://doi.org/10.1093/ehjci/jeac036
Andersen OS, Smiseth OA, Dokainish H et al (2017) Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 69:1937–1948. https://doi.org/10.1016/j.jacc.2017.01.058
Lessick J, Mutlak D, Efraim R et al (2022) Comparison between echocardiography and cardiac computed tomography in the evaluation of diastolic dysfunction and prediction of heart failure. Am J Cardiol 181:71–78. https://doi.org/10.1016/j.amjcard.2022.07.017
Somaratne JB, Whalley GA, Poppe KK et al (2009) Pseudonormal mitral filling is associated with similarly poor prognosis as restrictive filling in patients with heart failure and coronary heart disease: a systematic review and meta-analysis of prospective studies. J Am Soc Echocardiogr 22:494–498. https://doi.org/10.1016/j.echo.2009.02.003
Litwin SE, Zile MR (2020) Should we test for diastolic dysfunction? How and how often? JACC Cardiovasc Imaging 13:297–309. https://doi.org/10.1016/j.jcmg.2019.02.029
Barberato SH, Bucharles SGE, Sousa AM et al (2010) Prevalência e impacto prognóstico da disfunção diastólica na doença renal crônica em hemodiálise. Arq Bras Cardiol 94:457–462. https://doi.org/10.1590/S0066-782X2010005000016
De Lima JJG, Macedo TA, Gowdak LHW et al (2022) Diastolic and systolic left ventricular dysfunction and mortality in chronic kidney disease patients on haemodialysis. Nephrology 27:66–73. https://doi.org/10.1111/nep.13960
Suh SH, Oh TR, Choi HS et al (2022) Association of left ventricular diastolic dysfunction with cardiovascular outcomes in patients with pre-dialysis chronic kidney disease: findings from KNOW-CKD study. Front Cardiovasc Med 9:844312. https://doi.org/10.3389/fcvm.2022.844312
Playford D, Strange G, Celermajer DS et al (2021) Diastolic dysfunction and mortality in 436 360 men and women: the National Echo Database Australia (NEDA). Eur Heart J Cardiovasc Imaging 22:505–515. https://doi.org/10.1093/ehjci/jeaa253
Pezawas T, Burger AL, Binder T, Diedrich A (2020) Importance of diastolic function for the prediction of arrhythmic death: a prospective, observer-blinded, long-term study. Circ Arrhythm Electrophysiol 13:e007757. https://doi.org/10.1161/CIRCEP.119.007757
Kim K-H, She L, Lee KL et al (2020) Incremental prognostic value of echocardiography of left ventricular remodeling and diastolic function in STICH trial. Cardiovasc Ultrasound 18:17. https://doi.org/10.1186/s12947-020-00195-1
Metkus TS, Suarez-Pierre A, Crawford TC et al (2018) Diastolic dysfunction is common and predicts outcome after cardiac surgery. J Cardiothorac Surg 13:67. https://doi.org/10.1186/s13019-018-0744-3
Asami M, Lanz J, Stortecky S et al (2018) The impact of left ventricular diastolic dysfunction on clinical outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Interv 11:593–601. https://doi.org/10.1016/j.jcin.2018.01.240
Kampaktsis PN, Kokkinidis DG, Wong S-C et al (2017) The role and clinical implications of diastolic dysfunction in aortic stenosis. Heart 103:1481–1487. https://doi.org/10.1136/heartjnl-2017-311506
Ramos JG, Fyrdahl A, Wieslander B, et al (2020) Comprehensive cardiovascular magnetic resonance diastolic dysfunction grading shows very good agreement compared with echocardiography. JACC Cardiovasc Imaging S1936878X20306008. https://doi.org/10.1016/j.jcmg.2020.06.027
Paelinck BP, de Roos A, Bax JJ et al (2005) Feasibility of tissue magnetic resonance imaging. J Am Coll Cardiol 45:1109–1116. https://doi.org/10.1016/j.jacc.2004.12.051
Sharifov OF, Schiros CG, Aban I, et al (2016) Diagnostic accuracy of tissue Doppler index E/è for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: a systematic review and meta‐analysis. J Am Heart Assoc 5: https://doi.org/10.1161/JAHA.115.002530
Lancellotti P, Galderisi M, Edvardsen T et al (2017) Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-Filling study. Eur Heart J Cardiovasc Imaging 18:961–968. https://doi.org/10.1093/ehjci/jex067
Ran H, Schneider M, Pistritto AM et al (2019) Echocardiographic evaluation of left ventricular filling pressures in patients with pulmonary hypertension. Int J Cardiovasc Imaging 35:861–868. https://doi.org/10.1007/s10554-019-01528-6
D’Alto M, Romeo E, Argiento P et al (2015) Echocardiographic prediction of pre- versus postcapillary pulmonary hypertension. J Am Soc Echocardiogr 28:108–115. https://doi.org/10.1016/j.echo.2014.09.004
Geske JB, Sorajja P, Nishimura RA, Ommen SR (2007) Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation 116:2702–2708. https://doi.org/10.1161/CIRCULATIONAHA.107.698985
Mullens W, Borowski AG, Curtin RJ et al (2009) Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation 119:62–70. https://doi.org/10.1161/CIRCULATIONAHA.108.779223
Tomlinson S, Scalia GM, Appadurai V et al (2021) Left atrial reservoir strain provides incremental value to left atrial volume index for evaluation of left ventricular filling pressure. Echocardiography 38:1503–1513. https://doi.org/10.1111/echo.15157
Romano G, Magro S, Agnese V et al (2020) Echocardiography to estimate high filling pressure in patients with heart failure and reduced ejection fraction. ESC Heart Fail 7:2268–2277. https://doi.org/10.1002/ehf2.12748
Borde D, Joshi S, Jasapara A et al (2021) Left atrial strain as a single parameter to predict left ventricular diastolic dysfunction and elevated left ventricular filling pressure in patients undergoing off-pump coronary artery bypass grafting. J Cardiothorac Vasc Anesth 35:1618–1625. https://doi.org/10.1053/j.jvca.2020.11.066
Mandoli GE, Sisti N, Mondillo S, Cameli M (2020) Left atrial strain in left ventricular diastolic dysfunction: have we finally found the missing piece of the puzzle? Heart Fail Rev 25:409–417. https://doi.org/10.1007/s10741-019-09889-9
Cameli M, Lisi M, Mondillo S et al (2010) Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound 8:14. https://doi.org/10.1186/1476-7120-8-14
Sato K, Grant ADM, Negishi K et al (2017) Reliability of updated left ventricular diastolic function recommendations in predicting elevated left ventricular filling pressure and prognosis. Am Heart J 189:28–39. https://doi.org/10.1016/j.ahj.2017.03.022
Johansson B, Lundin F, Tegeback R, Bojö L (2019) E/a′ ratio is closely related to pulmonary vein flow profile in patients with normal ejection fraction. Scand Cardiovasc J 53:312–316. https://doi.org/10.1080/14017431.2019.1645348
Nagueh SF, Appleton CP, Gillebert TC et al (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10:165–193. https://doi.org/10.1093/ejechocard/jep007
Reiter C, Reiter G, Kräuter C et al (2023) Evaluation of left ventricular and left atrial volumetric function from native MR multislice 4D flow magnitude data. Eur Radiol. https://doi.org/10.1007/s00330-023-10017-3
Abdula G, Ramos JG, Marlevi D et al (2023) Non-invasive estimation of mean pulmonary artery pressure by cardiovascular magnetic resonance in under 2 min scan time. Eur Heart J Imaging Methods Pract 1:qyad014. https://doi.org/10.1093/ehjimp/qyad014
Varga‐Szemes A, Halfmann M, Schoepf UJ, et al (2022) Highly accelerated compressed‐sensing 4D flow for intracardiac flow assessment. J Magn Reson Imaging jmri.28484. https://doi.org/10.1002/jmri.28484
Hedström E, Bloch KM, Bergvall E et al (2010) Effects of gadolinium contrast agent on aortic blood flow and myocardial strain measurements by phase-contrast cardiovascular magnetic resonance. J Cardiovasc Magn Reson 12:70. https://doi.org/10.1186/1532-429X-12-70
Acknowledgements
The authors thank Ada Muellner, MS, for editing the manuscript.
Funding
Open access funding provided by Medical University of Graz. The study was funded by the Anniversary fund of the Austrian National Bank (Grant No. 17934) and by the ESR/EIBIR Seed Grant 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ursula Reiter.
Conflict of interest
Ursula Reiter is a member of the European Radiology Editorial Board. They have not taken part in the review or selection process of this article.
The authors of this manuscript declare relationships with the following companies: Gert Reiter is employee of Siemens Healthineers. The study was performed under a Master Research Agreement between the Medical University of Graz, Graz University of Technology, and Siemens Healthcare Diagnostics GmbH.
The other authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Gert Reiter) has significant statistical expertise. No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all patients in this study.
Ethical approval
Institutional Review Board approval was obtained.
Ethical committee
Medical University of Graz, Austria
ClinicalTrials.gov: NCT01728597, NCT03253835
Study subjects or cohorts overlap
• 4D flow data of 10 subjects of the study population were included in the definition of an algorithm for automated mitral valve vortex ring extraction from 4D flow MRI (Kräuter C et al, MRM 84 (2020). https://doi.org/10.1002/mrm.28361).
• The impact of breath-holding on cine real-time volumetric function parameters was analyzed previously from 56 subjects of this study population (Reiter C et al, Eur J Rad 141 (2021). https://doi.org/10.1016/j.ejrad.2021.109756).
• Volumetric function parameters of 60 subjects were analyzed from native multislice 4D flow data and compared to cine real-time imaging (Reiter C et al, Eur Radiol 2023; https://doi.org/10.1007/s00330-023-10017-3).
• 4D flow evaluation strategies for assessment of transmitral and myocardial velocities were analyzed in 60 subjects with echocardiography as reference; a manuscript is currently under review in the European Journal of Radiology.
Methodology
• prospective study
• diagnostic study
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reiter, C., Reiter, U., Kräuter, C. et al. MR 4D flow–derived left atrial acceleration factor for differentiating advanced left ventricular diastolic dysfunction. Eur Radiol (2023). https://doi.org/10.1007/s00330-023-10386-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-023-10386-9