Abstract
Purpose
This article aims to compare the diagnostic performance of 18-fluorodeoxyglucose ([18F]FDG) PET/CT and fibroblast activating protein inhibitor (FAPI) PET/CT in the assessment of primary tumors, lymph nodes, and distant metastases in lung cancer patients.
Methods
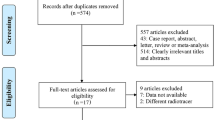
A systematic search was conducted on the Cochrane Library, Embase, and PubMed/MEDLINE databases from inception until November 1, 2022. Included studies assessed the use of FAPI PET/CT and [18F]FDG PET/CT in patients with lung cancer. The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was used to evaluate the risk of bias. A random variable model was used to analyze the diagnostic tests of the two imaging modalities.
Results
The sensitivity of FAPI PET/CT in detecting primary lung cancer lesions was 0.98 (95% CI: 0.88–1.00), while the sensitivity of [18F]FDG PET/CT was 0.99 (95% CI: 0.74–1.00). For the detection of metastatic lesions (lymph node metastases and distant metastases), FAPI PET/CT had a sensitivity of 0.99 (95% CI: 0.90–1.00), while the sensitivity of [18F]FDG PET/CT was 0.77 (95% CI: 0.66–0.85). However, the specificity of the two imaging modalities could not be assessed due to the lack of sufficient information on pertinent true negatives.
Conclusion
In the diagnosis of metastatic lung cancer lesions, FAPI PET/CT demonstrated a higher sensitivity compared to [18F]FDG PET/CT. Therefore, FAPI PET/CT may be considered an alternative imaging modality for the assessment of primary lung cancer tumors, lymph node metastases, and distant metastases.
Clinical relevance statement
FAPI may be an alternative to [18F]FDG in the assessment of primary lung cancer tumors, lymph node metastases, and distant metastases, which plays a very important role in treatment.
Key Points
• This article is to compare the performance of [18F]FDG PET/CT with FAPI PET/CT in the assessment of primary tumors, lymph nodes, and distant metastases in lung cancer.
• However, FAPI PET/CT has a higher sensitivity for the diagnostic assessment of metastatic lung cancer lesions.






Similar content being viewed by others
Abbreviations
- [18F]FDG:
-
18 Fluorodeoxyribose
- [68 Ga]Ga-FAPI:
-
68-Labeled fibroblast activating protein inhibitor
- 95% CI:
-
95% Confidence intervals
- CAFs:
-
Cancer-associated fibroblasts
- FAP:
-
Fibroblast activating protein
- LAC:
-
Lung adenocarcinoma
- LC:
-
Lung cancer
- NSCLC:
-
Nonsmall cell lung cancer
- P:
-
Prospective
- R:
-
Retrospective
- SUVmax:
-
Maximum standardized uptake
- TBR:
-
Target-to-background ratio
References
Siegel RL, Miller KD, Fuchs HE et al (2022) (2022) Cancer statistics. CA Cancer J Clin 72(1):7–33
Bray F, Ferlay J, Soerjomatarm I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Dickson JL, Horst C, Nair A et al (2022) Hesitancy around low-dose CT screening for lung cancer. Ann Oncol 33(1):34–41
Morozov SP, Gombolevskiy VA, Elizarov AB et al (2021) A simplified cluster model and a tool adapted for collaborative labeling of lung cancer CT scans. Comput Methods Programs Biomed 206:106111
de Koning HJ, van der Aalst CM, de Jong PA et al (2020) Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382(6):503–513
Zhang X, Yu Z (2021) Pathological analysis of hesperetin-derived small cell lung cancer by artificial intelligence technology under fiberoptic bronchoscopy. Math Biosci Eng 18(6):8538–8558
Skehan SJ, Issenman R, Mernagh J et al (1999) 18F-fluorodeoxyglucose positron tomography in diagnosis of paediatric inflammatory bowel disease. Lancet 354(9181):836–837
Instrumentation in positron emission tomography. Council on Scientific Affairs, (1988) Report of the Positron Emission Tomography Panel. JAMA 259(10):1531–1536
Scharko AM, Perlman SB, Pyzalski RW et al (2003) Whole-body positron emission tomography in patients with HIV-1 infection. Lancet 362(9388):959–961
Findlay M, Young H, Cunningham D et al (1996) Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol 14(3):700–708
Sazon DA, Santiago SM, Soo Hoo GW et al (1996) Fluorodeoxyglucose-positron emission tomography in the detection and staging of lung cancer. Am J Respir Crit Care Med 153(1):417–421
Schirrmeister H, Guhlmann A, Kotzerke J et al (1999) Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol 17(8):2381–2389
Lee JW, O JH, Choi M, Choi JY (2020) Impact of F-18 fluorodeoxyglucose PET/CT and PET/MRI on initial staging and changes in management of pancreatic ductal adenocarcinoma: a systemic review and meta-analysis. Diagnostics (Basel) 10(11):952
Cerfolio RJ, Ojha B, Bryant AS et al (2004) The accuracy of integrated PET-CT compared with dedicated PET alone for the staging of patients with nonsmall cell lung cancer. Ann Thorac Surg 78(3):1017–1023
Kalemkerian GP, Loo BW, Akerley W et al (2018) NCCN guidelines insights: small cell lung cancer, Version 2.2018. J Natl Compr Canc Netw 16(10):1171–1182
Konishi J, Yamazaki K, Tsukamoto E et al (2003) Mediastinal lymph node staging by FDG-PET in patients with non-small cell lung cancer: analysis of false-positive FDG-PET findings. Respiration 70(5):500–506
Al-Sarraf N, Gately K, Lucey J et al (2008) Lymph node staging by means of positron emission tomography is less accurate in non-small cell lung cancer patients with enlarged lymph nodes: analysis of 1,145 lymph nodes. Lung Cancer 60(1):62–68
Wu J, Deng H, Zhong H et al (2022) Comparison of (68)Ga-FAPI and (18)F-FDG PET/CT in the evaluation of patients with newly diagnosed non-small cell lung cancer. Front Oncol 12:924223
Huellner MW, de Galiza BF, Husmann L et al (2016) TNM staging of non-small cell lung cancer: comparison of PET/MR and PET/CT. J Nucl Med 57(1):21–26
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16(9):582–598
Kamali Zonouzi S, Pezeshki PS, Razi S et al (2022) Cancer-associated fibroblasts in colorectal cancer. Clin Transl Oncol 24(5):757–769
Mezawa Y, Orimo A (2022) Phenotypic heterogeneity, stability and plasticity in tumor-promoting carcinoma-associated fibroblasts. FEBS J 289(9):2429–2447
Koerber SA, Staudinger F, Kratochwil C et al (2020) The role of (68)Ga-FAPI PET/CT for patients with malignancies of the lower gastrointestinal tract: first clinical experience. J Nucl Med 61(9):1331–1336
Röhrich M, Loktev A, Wefers AK et al (2019) IDH-wildtype glioblastomas and grade III/IV IDH-mutant gliomas show elevated tracer uptake in fibroblast activation protein-specific PET/CT. Eur J Nucl Med Mol Imaging 46(12):2569–2580
Huang D, Wu J, Zhong H et al (2023) [(68)Ga]Ga-FAPI PET for the evaluation of digestive system tumors: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 50(3):908–920
Zhou X, Wang S, Xu X et al (2022) Higher accuracy of [(68) Ga]Ga-DOTA-FAPI-04 PET/CT comparing with 2-[(18)F]FDG PET/CT in clinical staging of NSCLC. Eur J Nucl Med Mol Imaging 49(8):2983–2993
Wang L, Tang G, Hu K et al (2022) Comparison of (68)Ga-FAPI and (18)F-FDG PET/CT in the evaluation of advanced lung cancer. Radiology 303(1):191–199
McInnes MDF, Moher D, Thombs BD et al (2018) Preferred Reporting Items for a Systematic Review and Meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA Statement. JAMA 319(4):388–396
Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
von Hippel PT (2015) The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol 15:35
Giesel FL, Kratochwil C, Lindner T et al (2019) (68)Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med 60(3):386–392
Li Y, Lin X, Li Y et al (2022) Clinical utility of F-18 labeled fibroblast activation protein inhibitor (FAPI) for primary staging in lung adenocarcinoma: a prospective study. Mol Imaging Biol 24(2):309–320
Zhu L, Yin G, Chen W et al (2019) Correlation between EGFR mutation status and F(18) -fluorodeoxyglucose positron emission tomography-computed tomography image features in lung adenocarcinoma. Thorac Cancer 10(4):659–664
Li ZM, Ding ZP, Luo QQ et al (2013) Prognostic significance of the extent of lymph node involvement in stage II-N1 non-small cell lung cancer. Chest 144(4):1253–1260
Serfling S, Zhi Y, Schirbel A et al (2021) Improved cancer detection in Waldeyer’s tonsillar ring by (68)Ga-FAPI PET/CT imaging. Eur J Nucl Med Mol Imaging 48(4):1178–1187
Can C, Kepenek F, Kömek H et al (2022) Comparison of 18 F-FDG PET/CT and 68 Ga-FAPI-04 PET/CT in patients with non-small cell lung cancer. Nucl Med Commun 43(10):1084–1091
Wei Y, Cheng K, Fu Z et al (2022) [(18)F]AlF-NOTA-FAPI-04 PET/CT uptake in metastatic lesions on PET/CT imaging might distinguish different pathological types of lung cancer. Eur J Nucl Med Mol Imaging 49(5):1671–1681
Acknowledgements
This study was supported by the Luzhou Municipal People’s Government-Southwest Medical University Science and Technology Strategic Cooperation Fund.
Funding
This study has received funding by the Luzhou Municipal People’s Government-Southwest Medical University Science and Technology Strategic Cooperation Fund (No. 2020LZXNYDJ12); Sichuan Provincial Medical Research Project Program (No. S21004); Southwest Medical University Innovation and Entrepreneurship Training Program (No.S202210632248).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ping Zhou.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Delong Huang kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was not required for this study because we statistically analyzed other people’s experiments and it was registered with PROSPERO9 (CRD42022374792; available at https://www.crd.york.ac.uk/PROSPERO).
Ethical approval
Institutional Review Board approval was not required because it was a systematic review and meta-analysis.
Study subjects or cohorts overlap
Some study subjects or cohorts have not been previously reported.
Methodology and design-location
• prospective
• diagnostic study
• Performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiang Zhan and Ping Zhou are co-corresponding authors; they contributed equally to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Q., Huang, D., Wu, J. et al. Performance of [18F]FDG PET/CT versus FAPI PET/CT for lung cancer assessment: a systematic review and meta-analysis. Eur Radiol 34, 1077–1085 (2024). https://doi.org/10.1007/s00330-023-10013-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10013-7