Abstract
Objectives
Diffusion prepared pseudo-continuous arterial spin labeling (DP-pCASL) is a newly proposed MRI method to noninvasively measure the function of the blood–brain barrier (BBB). We aim to investigate whether the water exchange rate across the BBB, estimated with DP-pCASL, is changed in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), and to analyze the association between the BBB water exchange rate and MRI/clinical features of these patients.
Methods
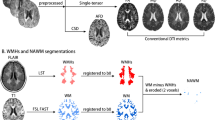
Forty-one patients with CADASIL and thirty-six age- and sex-matched controls were scanned with DP-pCASL MRI to estimate the BBB water exchange rate (kw). The MRI lesion burden, the modified Rankin scale (mRS), and the neuropsychological scales were also examined. The association between kw and MRI/clinical features was analyzed.
Results
Compared with that in the controls, kw in patients with CADASIL was decreased at normal-appearing white matter (NAWM) (t = − 4.742, p < 0.001), cortical gray matter (t = − 5.137, p < 0.001), and deep gray matter (t = − 3.552, p = 0.001). After adjustment for age, gender, and arterial transit time, kw at NAWM was negatively associated with the volume of white matter hyperintensities (β = − 0.754, p = 0.001), whereas decreased kw at NAWM was independently associated with an increased risk of abnormal mRS scale (OR = 1.058, 95% CI: 1.013–1.106, p = 0.011) in these patients.
Conclusions
This study found that the BBB water exchange rate was decreased in patients with CADASIL. The decreased BBB water exchange rate was associated with an increased MRI lesion burden and functional dependence of the patients, suggesting the involvement of BBB dysfunction in the pathogenesis of CADASIL.
Clinical relevance statement
DP-pCASL reveals BBB dysfunction in patients with CADASIL. The decreased BBB water exchange rate is associated with MRI lesion burden and functional dependence, indicating the potential of DP-pCASL as an evaluation method for disease severity.
Key Points
• DP-pCASL reveals blood–brain barrier dysfunction in patients with CADASIL.
• Decreased BBB water exchange rate, an indicator of BBB dysfunction detected by DP-pCASL, was associated with MRI/clinical features of patients with CADASIL.
• DP-pCASL can be used as an evaluation method to assess the severity of disease in patients with CADASIL.


Similar content being viewed by others
Abbreviations
- ASL:
-
Arterial spin labeling
- ATT:
-
Arterial transit time
- BBB:
-
Blood–brain barrier
- CADASIL:
-
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- CBF:
-
Cerebral blood flow
- CGM:
-
Cortical gray matter
- CMBs:
-
Cerebral microbleeds
- DCE-MRI:
-
Dynamic contrast-enhanced MRI
- DGM:
-
Deep gray matter
- DP-pCASL:
-
Diffusion prepared pseudo-continuous ASL
- MD-pCASL:
-
Multi-delay pseudo-continuous ASL
- MMSE:
-
Mini-Mental State Examination
- MoCA:
-
Montreal Cognitive Assessment
- mRS:
-
Modified Rankin scale
- NAWM:
-
Normal-appearing white matter
- ROIs:
-
Regions of interest
- SAS:
-
Zung’s Self-Rating Anxiety Scale
- SDMT:
-
Symbol Digit Modality Test
- SDS:
-
Zung’s Self-Rating Depression Scale
- TMT:
-
Trail Making Test
- WMH:
-
White matter hyperintensity
References
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19:1584–1596
Marques F, Sousa JC, Sousa N, Palha JA (2013) Blood-brain-barriers in aging and in Alzheimer’s disease. Mol Neurodegener 8:38
Dickie BR, Parker GJM, Parkes LM (2020) Measuring water exchange across the blood-brain barrier using MRI. Prog Nucl Magn Reson Spectrosc 116:19–39
Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB (2017) Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 16:564–570
St Lawrence KS, Owen D, Wang DJ (2012) A two-stage approach for measuring vascular water exchange and arterial transit time by diffusion-weighted perfusion MRI. Magn Reson Med 67:1275–1284
Shao X, Ma SJ, Casey M, D’Orazio L, Ringman JM, Wang DJJ (2019) Mapping water exchange across the blood-brain barrier using 3D diffusion-prepared arterial spin labeled perfusion MRI. Magn Reson Med 81:3065–3079
Gold BT, Shao X, Sudduth TL et al (2021) Water exchange rate across the blood-brain barrier is associated with CSF amyloid-β 42 in healthy older adults. Alzheimers Dement 17:2020–2029
Shao X, Jann K, Ma SJ et al (2020) Comparison between blood-brain barrier water exchange rate and permeability to gadolinium-based contrast agent in an elderly cohort. Front Neurosci 14:571480
Dickie BR, Vandesquille M, Ulloa J, Boutin H, Parkes LM, Parker GJM (2019) Water-exchange MRI detects subtle blood-brain barrier breakdown in Alzheimer’s disease rats. Neuroimage 184:349–358
Uchida Y, Kan H, Sakurai K et al (2022) APOE ɛ4 dose associates with increased brain iron and β-amyloid via blood-brain barrier dysfunction. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2021-328519
Di Donato I, Bianchi S, De Stefano N et al (2017) Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) as a model of small vessel disease: update on clinical, diagnostic, and management aspects. BMC Med 15:41
Sun C, Wu Y, Ling C et al (2022) Reduced blood flow velocity in lenticulostriate arteries of patients with CADASIL assessed by PC-MRA at 7T. J Neurol Neurosurg Psychiatry 93:451–452
Uchida Y, Kan H, Sakurai K et al (2020) Iron leakage owing to blood-brain barrier disruption in small vessel disease CADASIL. Neurology 95:e1188–e1198
Walsh J, Tozer DJ, Sari H et al (2021) Microglial activation and blood-brain barrier permeability in cerebral small vessel disease. Brain 144:1361–1371
Rajani RM, Ratelade J, Domenga-Denier V et al (2019) Blood brain barrier leakage is not a consistent feature of white matter lesions in CADASIL. Acta Neuropathol Commun 7:187
Ling C, Zhang Z, Wu Y et al (2019) Reduced venous oxygen saturation associates with increased dependence of patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: a 7.0-T magnetic resonance imaging study. Stroke 50:3128–3134
Yang K, Shen B, Li DK et al (2018) Cognitive characteristics in Chinese non-demented PD patients based on gender difference. Transl Neurodegener 7:16
Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG (1992) Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 4:134–139
Dunstan DA, Scott N, Todd AK (2017) Screening for anxiety and depression: reassessing the utility of the Zung scales. BMC Psychiatry 17:329
Chappell MA, Groves AR, Whitcher B, Woolrich MW (2008) Variational Bayesian inference for a nonlinear forward model. IEEE Trans Signal Process 57:223–236
Yan L, Liu CY, Wong KP et al (2018) Regional association of pCASL-MRI with FDG-PET and PiB-PET in people at risk for autosomal dominant Alzheimer’s disease. Neuroimage Clin 17:751–760
Wu Y, Ji F, Chong YF, Chen L-HC, Zhou JH (2022) Deeply supervised network for white matter hyperintensities segmentation with transfer learning. Medical Imaging with Deep Learning,
Wardlaw JM, Smith EE, Biessels GJ et al (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12:822–838
Gregoire SM, Chaudhary UJ, Brown MM et al (2009) The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology 73:1759–1766
Li Y, Ying Y, Yao T et al (2023) Decreased water exchange rate across blood-brain barrier in hereditary cerebral small vessel disease. Brain. https://doi.org/10.1093/brain/awac500
Nelis P, Kleffner I, Burg MC et al (2018) OCT-angiography reveals reduced vessel density in the deep retinal plexus of CADASIL patients. Sci Rep 8:8148
Ling C, Fang X, Kong Q et al (2019) Lenticulostriate arteries and basal ganglia changes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, a high-field MRI study. Front Neurol 10:870
Chu H, Huang C, Ding H et al (2016) Aquaporin-4 and cerebrovascular diseases. Int J Mol Sci 17
Salman MM, Kitchen P, Halsey A et al (2022) Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 145:64–75
Hase Y, Chen A, Bates LL et al (2018) Severe white matter astrocytopathy in CADASIL. Brain Pathol 28:832–843
Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG (2018) The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol 135:387–407
Monet-Leprêtre M, Haddad I, Baron-Menguy C et al (2013) Abnormal recruitment of extracellular matrix proteins by excess Notch3 ECD: a new pathomechanism in CADASIL. Brain 136:1830–1845
Ferrante EA, Cudrici CD, Boehm M (2019) CADASIL: new advances in basic science and clinical perspectives. Curr Opin Hematol 26:193–198
Yao M, Jouvent E, During M et al (2012) Extensive white matter hyperintensities may increase brain volume in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 43:3252–3257
Yu X, Yin X, Hong H et al (2021) Increased extracellular fluid is associated with white matter fiber degeneration in CADASIL: in vivo evidence from diffusion magnetic resonance imaging. Fluids Barriers CNS 18:29
Spann SM, Shao X, Wang DJ et al (2020) Robust single-shot acquisition of high resolution whole brain ASL images by combining time-dependent 2D CAPIRINHA sampling with spatio-temporal TGV reconstruction. Neuroimage 206:116337
Acknowledgements
The authors acknowledge Yue Wu, Dongbiao Sun, and Dixuan Wu (Institute of Biophysics, Chinese Academy of Sciences) for their contribution to MRI data acquisition. The authors also appreciate the support of Weili Yang (Department of Neurology, Peking University First Hospital) for administrative assistance. Partial technical support for the imaging optimization was provided by Dr. Jing An (Siemens Shenzhen Magnetic Resonance Ltd).
Funding
This study has received funding from the National Natural Science Foundation of China (82101355, 81961128030, 82001804), Scientific Research Seed Fund of the Peking University First Hospital (2021SF06), Key Technologies Research and Development Program of China (2016YFC1300605), National Science and Technology Innovation 2030 Major Program (2022ZD0211901), Youth Innovation Promotion Association of the CAS (2022093), US National Institutes of Health (R01NS114382), and Ministry of Science and Technology of China (2019YFA0707103).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Zihao Zhang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
This study was approved by the Institutional Review Board and Ethics Committee of Peking University First Hospital.
Methodology
• cross-sectional study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ling, C., Zhang, J., Shao, X. et al. Diffusion prepared pseudo-continuous arterial spin labeling reveals blood–brain barrier dysfunction in patients with CADASIL. Eur Radiol 33, 6959–6969 (2023). https://doi.org/10.1007/s00330-023-09652-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09652-7