Abstract
Objectives
Current surgical policy recommends comprehensive excision of tumorous calcifications in breast cancer patients following neoadjuvant chemotherapy (NAC) regardless of MRI outcomes, despite MRI defining tumor response superior to mammography. The current study examines MRI prediction of response in tumors with vs without calcifications, using post-NAC surgical pathology as the standard of reference.
Methods
Retrospective analysis of 114 NAC patients between 2011 and 2018 including demographics, mammography, 3 T-MRI, and pathology compared two sub-groups: without (n = 62) or with (n = 52) mammographic calcifications. In the calcification cohort, the mammographic extent of calcifications and MRI enhancement overlapped. MRI prediction of response to NAC was correlated with pathology. Two-tailed paired T and Fisher’s exact tests and Cohen’s kappa coefficient were applied for analysis.
Results
There was no significant difference between the two sub-groups regarding demographics. Tumors demonstrated equivalent features regarding size, lymph node involvement, and DCIS component. ER-negative/HER2-positive tumors more commonly exhibited calcifications (33% n = 17 calcified vs 13% n = 8 non-calcified; p < 0.05); triple negative pathology rarely calcified (6% n = 3 calcified vs 33% n = 20 non-calcified; p < 0.05). NME was more common with calcifications (62% n = 32 calcified vs 29% n = 18 non-calcified; p < 0.05) and mass enhancement without (90% n = 56 non-calcified vs 81% n = 42 calcified; p < 0.05). Both groups responded similarly to NAC (pCR = 37% non-calcified vs 38% calcified); response on MRI equally correlated with pathology (69% both subgroups; p = 0.988).
Conclusion
We propose utilizing post-NAC MRI findings rather than mammography in planning surgery, as MRI prediction is independent of the presence or absence of calcifications. Prospective studies to evaluate this approach are warranted.
Key Points
• No difference was found in demographic, clinical, pathology, or imaging characteristics between patients with or without tumoral calcifications on mammography prior to neoadjuvant chemotherapy.
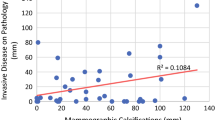
• Residual mammographic calcifications are inadequate predictors of residual invasive disease. MRI accurately recognized complete response and correctly correlated with post-treatment surgical pathology in 69% of patients, regardless of the presence or absence of mammographic calcifications.
• We propose utilizing post-NAC MRI findings rather than mammography in planning post-NAC surgery, as MRI prediction of response is independent of the presence or absence of calcifications.


Similar content being viewed by others
Abbreviations
- BCS:
-
Breast-conserving surgery
- DCIS:
-
Ductal carcinoma in situ
- IDC:
-
Invasive ductal carcinoma
- ILC:
-
Invasive lobular carcinoma
- LABC:
-
Locally advanced breast cancer
- MRI:
-
Magnetic resonance imaging
- NAC:
-
Neoadjuvant chemotherapy
- NME:
-
Non-mass enhancement
- pCR:
-
Pathologic complete response
- rCR:
-
Radiologic complete response
References
Mieog JS, van der Hage JA, van de Velde CJ (2007) Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 94(10):1189–1200
Untch M, Konecny GE, Paepke S, Minckwitz GV (2014) Current and future role of neoadjuvant therapy for breast cancer. Breast 23(5):526–537
Mamtani A,Sevilimedu V, Le T et al (2022) Is local recurrence higher among patients who downstage to breast conservation after neoadjuvant chemotherapy? Cancer 128(3):471–478
Mamtani A, Barrio AV, King TA et al (2016) How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol 23(11):3467–3474
Montagna G, Mamtani A, Knezevic A, Brogi E, Barrio AV, Morrow M (2020) Selecting node-positive patients for axillary downstaging with neoadjuvant chemotherapy. Ann Surg Oncol 27(11):4515–4522
Kong X, Moran Ms, Zhang N, Haffty B, Yang Q (2011) Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 47(14):2084–2090
Fowler AM, Mankoff DA, Joe BN (2017) Imaging neoadjuvant therapy response in breast cancer. Radiology 285(2):358–375
Hylton NM, Blume JD, Bernreuter Wk et al (2012) Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy–results from ACRIN 6657/I-SPY TRIAL. Radiology 263(3):663–672
Romeo V, Accardo G, Perillo T et al (2021) Assessment and prediction of response to neoadjuvant chemotherapy in breast cancer: a comparison of imaging modalities and future perspectives. Cancers (Basel) 13(14)
Mann RM, Kuhl CK, Kinkel K, Boetes C (2008) Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol 18(7):1307–1318
Slanetz PJ, Moy L, Baron P et al (2017) ACR Appropriateness Criteria(®) monitoring response to neoadjuvant systemic therapy for breast cancer. J Am Coll Radiol 14(11s):S462-s475
Croshaw R, Shapiro-Wright H, Svensson E, Erb K, Julian T (2011) Accuracy of clinical examination, digital mammogram, ultrasound, and MRI in determining postneoadjuvant pathologic tumor response in operable breast cancer patients. Ann Surg Oncol 18(11):3160–3163
de Los Santos J, Cantor A, Amos KD et al (2013) Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer Translational Breast Cancer Research Consortium trial 017. Cancer 119(10): 1776–83
Choi WJ, Kim HH, Cha JH, Shin HJ, Chae EY, Yoon GY (2019) Complete response on MR imaging after neoadjuvant chemotherapy in breast cancer patients: factors of radiologic-pathologic discordance. Eur J Radiol 118:114–121
Gu YL, Pan S-M, Ren J, Yang ZX, Jiang GQ (2017) Role of magnetic resonance imaging in detection of pathologic complete remission in breast cancer patients treated with neoadjuvant chemotherapy: a meta-analysis. Clin Breast Cancer 17(4):245–255
Kim J, Han BK, Ko EY, Ko ES, Choi JS, Park KW (2022) Prediction of pathologic complete response on MRI in patients with breast cancer receiving neoadjuvant chemotherapy according to molecular subtypes. Eur Radiol 32:4056–4066
Adrada BE, Huo L, Lane DL, Arribas EM, Resetkova E, Yang W (2015) Histopathologic correlation of residual mammographic microcalcifications after neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol 22(4):1111–1117
Feliciano Y, Mamtani A, Morrow M, Stempel MM, Patil S, Jochelson MS (2017) Do calcifications seen on mammography after neoadjuvant chemotherapy for breast cancer always need to be excised? Ann Surg Oncol 24(6):1492–1498
Li JJ, Chen C, Gu Y, et al (2014) The role of mammographic calcification in the neoadjuvant therapy of breast cancer imaging evaluation. PLoS One 9(2):e88853
An YY, Kim SH, Kang BJ (2017) Residual microcalcifications after neoadjuvant chemotherapy for locally advanced breast cancer: comparison of the accuracies of mammography and MRI in predicting pathological residual tumor. World J Surg Oncol 15(1):198
Slonimsky E, Azraq Y, Gomori JM, Fisch S, Kleinman TA, Sella T (2020) Intravenous line phase-wrap artifact at bilateral axial 3-T breast MRI: identification, analysis, and solution. Radiol Imaging Cancer 2(6):e200004
Lakhani SR, E.I., Schnitt SJ, Tan PH, van de Vijver MJ, WHO classification of tumours of the breast 4th edition. 4 ed. WHO Classification of Tumours. 2012, Lyon, France: WHO IARC.
Elston CW (1991) Ellis IO Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–10
Sinha PS, Bendall S, Bates T (2000) Does routine grading of invasive lobular cancer of the breast have the same prognostic significance as for ductal cancers? Eur J Surg Oncol 26(8):733–737
Rakha EA, El-Sayed ME, Menon S, Green AR, Lee AH, Ellis IO (2008) Histologic grading is an independent prognostic factor in invasive lobular carcinoma of the breast. Breast Cancer Res Treat 111(1):121–127
Morrow M, Khan AJ (2020) Locoregional management after neoadjuvant chemotherapy. J Clin Oncol 38(20):2281–2289
Marinovich ML, Houssami N, Macaskill P et al (2013) Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst 105(5):321–333
van la RF Parra, HM Kuerer 2016 Selective elimination of breast cancer surgery in exceptional responders: historical perspective and current trials. Breast Cancer Res 18(1):28
Heil J, Kuerer HM, Pfob A et al (2020) Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: current evidence and future challenges. Ann Oncol 31(1):61–71
Thompson BM, Chala LF, Shimizu C et al (2022) Pre-treatment MRI tumor features and post-treatment mammographic findings: may they contribute to refining the prediction of pathologic complete response in post-neoadjuvant breast cancer patients with radiologic complete response on MRI? Eur Radiol 32(3):1663–1675
Kuhl CK (2009) Why do purely intraductal cancers enhance on breast MR images? Radiology 253(2):281–283
Kuhl CK, Schrading S, Bieling HB et al (2007) MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet 370(9586):485–492
Rauch GM, Kuerer HM, Adrada B et al (2018) Biopsy feasibility trial for breast cancer pathologic complete response detection after neoadjuvant chemotherapy: imaging assessment and correlation endpoints. Ann Surg Oncol 25(7):1953–1960
Acknowledgements
This study was performed as an MD dissertation to fulfill requirements at the Hadassah Hebrew University Medical School. We would like to thank Ms Tali Bdolah-Abram from the Hadassah Hebrew University Medical School for her statistical consultation and guidance.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Tamar Sella.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
We would like to thank Ms Tali Bdolah-Abram from the Hadassah Hebrew University Medical School for her statistical consultation and guidance.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained (Hadassah Hebrew University Medical Center Institutional Review Board, ref. 0299–18-HMO).
Study subjects or cohorts overlap
No overlap in study subjects or cohorts.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sella, T., Simor, B., Adler - Levy, Y. et al. MRI prediction of neoadjuvant chemotherapy response is equivalent in patients with or without mammographic calcifications: a step towards adapting surgical approach?. Eur Radiol 33, 7168–7177 (2023). https://doi.org/10.1007/s00330-023-09640-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09640-x