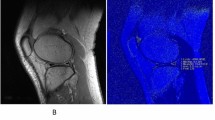
Abstract
Objective
To assess the detection of changes in knee cartilage and meniscus of amateur marathon runners before and after long-distance running using a 3D ultrashort echo time MRI sequence with magnetization transfer preparation (UTE-MT).
Methods
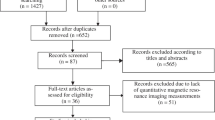
We recruited 23 amateur marathon runners (46 knees) in this prospective cohort study. MRI scans using UTE-MT and UTE-T2* sequences were performed pre-race, 2 days post-race, and 4 weeks post-race. UTE-MT ratio (UTE-MTR) and UTE-T2* were measured for knee cartilage (eight subregions) and meniscus (four subregions). The sequence reproducibility and inter-rater reliability were also investigated.
Results
Both the UTE-MTR and UTE-T2* measurements showed good reproducibility and inter-rater reliability. For most subregions of cartilage and meniscus, the UTE-MTR values decreased 2 days post-race and increased after 4 weeks of rest. Conversely, the UTE-T2* values increased 2 days post-race and decreased after 4 weeks. The UTE-MTR values in lateral tibial plateau, central medial femoral condyle, and medial tibial plateau showed a significant decrease at 2 days post-race compared to the other two time points (p < 0.05). By comparison, no significant UTE-T2* changes were found for any cartilage subregions. For meniscus, the UTE-MTR values in medial posterior horn and lateral posterior horn regions at 2 days post-race were significantly lower than those at pre-race and 4 weeks post-race (p < 0.05). By comparison, only the UTE-T2* values in medial posterior horn showed a significant difference.
Conclusions
UTE-MTR is a promising method for the detection of dynamic changes in knee cartilage and meniscus after long-distance running.
Key Points
• Long-distance running causes changes in the knee cartilage and meniscus.
• UTE-MT monitors dynamic changes of knee cartilage and meniscal non-invasively.
• UTE-MT is superior to UTE-T2* in monitoring dynamic changes in knee cartilage and meniscus.





Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- CI:
-
Confidence intervals
- CLFC:
-
Central lateral femoral condyle
- CMFC:
-
Central medial femoral condyle
- ICC:
-
Intraclass correlation coefficient
- LAH:
-
Lateral anterior horn
- LPH:
-
Lateral posterior horn
- LTP:
-
Lateral tibial plateau
- MAH:
-
Medial anterior horn
- MPH:
-
Medial posterior horn
- MTP:
-
Medial tibial plateau
- OA:
-
Osteoarthritis
- PAT:
-
Patella
- PLFC:
-
Posterior lateral femoral condyle
- PMFC:
-
Posterior medial femoral condyle
- ROI:
-
Regions of interest
- RRMI:
-
Running-related musculoskeletal injury
- SNR:
-
Signal-to-noise ratio
- TRO:
-
Trochlea
- UTE:
-
Ultrashort echo time
- UTE-MT:
-
Ultrashort echo time magnetization transfer
- UTE-MTR:
-
Ultrashort echo time magnetization transfer ratio
References
Lopes AD, HespanholJúnior LC, Yeung SS, Costa LO (2012) What are the main running-related musculoskeletal injuries? A Systematic Review. Sports Med 42:891–905
Kakouris N, Yener N, Fong DTP (2021) A systematic review of running-related musculoskeletal injuries in runners. J Sport Health Sci 10:513–522
Haskell WL, Lee IM, Pate RR et al (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39:1423–1434
Wilusz RE, Sanchez-Adams J, Guilak F (2014) The structure and function of the pericellular matrix of articular cartilage. Matrix Biol 39:25–32
Greene GW, Banquy X, Lee DW, Lowrey DD, Yu J, Israelachvili JN (2011) Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc Natl Acad Sci U S A 108:5255–5259
Stehling C, Luke A, Stahl R et al (2011) Meniscal T1rho and T2 measured with 3.0T MRI increases directly after running a marathon. Skeletal Radiol 40:725–735
Bashir A, Gray ML, Boutin RD, Burstein D (1997) Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology 205:551–558
Rauscher I, Stahl R, Cheng J et al (2008) Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology 249:591–600
Fang L, Ye Y, Tan X, Huang L, He Y (2021) Overloading stress–induced progressive degeneration and self-repair in condylar cartilage. Ann N Y Acad Sci 1503:72–87
Hunter DJ, Guermazi A, Lo GH et al (2011) Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage 19:990–1002
Englund M, Guermazi A, Gale D et al (2008) Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med 359:1108–1115
Roemer FW, Kwoh CK, Hayashi D, Felson DT, Guermazi A (2018) The role of radiography and MRI for eligibility assessment in DMOAD trials of knee OA. Nat Rev Rheumatol 14:372–380
Eijgenraam SM, Bovendeert FAT, Verschueren J et al (2019) T(2) mapping of the meniscus is a biomarker for early osteoarthritis. Eur Radiol 29:5664–5672
Wang A, Pedoia V, Su F et al (2016) MR T1ρ and T2 of meniscus after acute anterior cruciate ligament injuries. Osteoarthritis Cartilage 24:631–639
Kajabi AW, Casula V, Nissi MJ et al (2018) Assessment of meniscus with adiabatic T(1ρ) and T(2ρ) relaxation time in asymptomatic subjects and patients with mild osteoarthritis: a feasibility study. Osteoarthritis Cartilage 26:580–587
Zarins ZA, Bolbos RI, Pialat JB et al (2010) Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage 18:1408–1416
Li X, Pai A, Blumenkrantz G et al (2009) Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med 61:1310–1318
Chang EY, Du J, Bae WC, Chung CB (2015) Qualitative and Quantitative Ultrashort Echo Time Imaging of Musculoskeletal Tissues. Semin Musculoskelet Radiol 19:375–386
Shao H, Pauli C, Li S et al (2017) Magic angle effect plays a major role in both T1rho and T2 relaxation in articular cartilage. Osteoarthritis Cartilage 25:2022–2030
Wu M, Ma YJ, Kasibhatla A et al (2020) Convincing evidence for magic angle less-sensitive quantitative T(1ρ) imaging of articular cartilage using the 3D ultrashort echo time cones adiabatic T(1ρ) (3D UTE cones-AdiabT(1ρ) ) sequence. Magn Reson Med 84:2551–2560
Wu M, Ma Y, Wan L et al (2020) Magic angle effect on adiabatic T(1ρ) imaging of the Achilles tendon using 3D ultrashort echo time cones trajectory. NMR Biomed 33:e4322
Xia Y (2000) Magic-angle effect in magnetic resonance imaging of articular cartilage: a review. Invest Radiol 35:602–621
McWalter EJ, Gold GE (2012) UTE T2∗ mapping detects sub-clinical meniscus degeneration. Osteoarthritis Cartilage 20:471–472
Du J, Takahashi AM, Chung CB (2009) Ultrashort TE spectroscopic imaging (UTESI): application to the imaging of short T2 relaxation tissues in the musculoskeletal system. J Magn Reson Imaging 29:412–421
High RA, Ji Y, Ma YJ et al (2019) In vivo assessment of extracellular pH of joint tissues using acidoCEST-UTE MRI. Quant Imaging Med Surg 9:1664–1673
Williams AA, Titchenal MR, Andriacchi TP, Chu CR (2018) MRI UTE-T2* profile characteristics correlate to walking mechanics and patient reported outcomes 2 years after ACL reconstruction. Osteoarthritis Cartilage 26:569–579
Chang EY, Du J, Chung CB (2015) UTE imaging in the musculoskeletal system. J Magn Reson Imaging 41:870–883
Du J, Carl M, Diaz E et al (2010) Ultrashort TE T1rho (UTE T1rho) imaging of the Achilles tendon and meniscus. Magn Reson Med 64:834–842
Afsahi AM, Sedaghat S, Moazamian D et al (2022) Articular Cartilage Assessment Using Ultrashort Echo Time MRI: A Review. Front Endocrinol (Lausanne) 13:892961
Zhang X, Ma YJ, Wei Z et al (2021) Macromolecular fraction (MMF) from 3D ultrashort echo time cones magnetization transfer (3D UTE-Cones-MT) imaging predicts meniscal degeneration and knee osteoarthritis. Osteoarthritis Cartilage 29:1173–1180
Yang J, Shao H, Ma Y et al (2020) Quantitative ultrashort echo time magnetization transfer (UTE-MT) for diagnosis of early cartilage degeneration: comparison with UTE-T2* and T2 mapping. Quant Imaging Med Surg 10:171–183
Afsahi AM, Ma Y, Jang H et al (2022) Ultrashort Echo Time Magnetic Resonance Imaging Techniques: Met and Unmet Needs in Musculoskeletal Imaging. J Magn Reson Imaging 55:1597–1612
Ma YJ, Carl M, Searleman A, Lu X, Chang EY, Du J (2018) 3D adiabatic T(1ρ) prepared ultrashort echo time cones sequence for whole knee imaging. Magn Reson Med 80:1429–1439
Ma YJ, Chang EY, Carl M, Du J (2018) Quantitative magnetization transfer ultrashort echo time imaging using a time-efficient 3D multispoke Cones sequence. Magn Reson Med 79:692–700
Shao H, Chang EY, Pauli C et al (2016) UTE bi-component analysis of T2* relaxation in articular cartilage. Osteoarthritis Cartilage 24:364–373
Ma YJ, Shao H, Du J, Chang EY (2016) Ultrashort echo time magnetization transfer (UTE-MT) imaging and modeling: magic angle independent biomarkers of tissue properties. NMR Biomed 29:1546–1552
Zhu Y, Cheng X, Ma Y et al (2018) Rotator cuff tendon assessment using magic-angle insensitive 3D ultrashort echo time cones magnetization transfer (UTE-Cones-MT) imaging and modeling with histological correlation. J Magn Reson Imaging 48:160–168
Jerban S, Ma Y, Namiranian B et al (2019) Age-related decrease in collagen proton fraction in tibial tendons estimated by magnetization transfer modeling of ultrashort echo time magnetic resonance imaging (UTE-MRI). Sci Rep 9:17974
von Drygalski A, Barnes RFW, Jang H et al (2019) Advanced magnetic resonance imaging of cartilage components in haemophilic joints reveals that cartilage hemosiderin correlates with joint deterioration. Haemophilia 25:851–858
Jerban S, Hananouchi T, Ma Y et al (2022) Correlation between the elastic modulus of anterior cruciate ligament (ACL) and quantitative ultrashort echo time (UTE) magnetic resonance imaging. J Orthop Res 40:2330–2339
Williams A, Qian Y, Golla S, Chu CR (2012) UTE-T2∗ mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage 20:486–494
Wilms LM, Radke KL, Latz D et al (2022) UTE-T2* versus conventional T2* mapping to assess posterior cruciate ligament ultrastructure and integrity-an in-situ study. Quant Imaging Med Surg 12:4190–4201
Warth RJ, Zandiyeh P, Rao M et al (2020) Quantitative Assessment of In Vivo Human Anterior Cruciate Ligament Autograft Remodeling: A 3-Dimensional UTE-T2* Imaging Study. Am J Sports Med 48:2939–2947
Ma YJ, Chang EY, Bydder GM, Du J (2016) Can ultrashort-TE (UTE) MRI sequences on a 3-T clinical scanner detect signal directly from collagen protons: freeze-dry and D2 O exchange studies of cortical bone and Achilles tendon specimens. NMR Biomed 29:912–917
Li W, Hong L, Hu L, Magin RL (2010) Magnetization transfer imaging provides a quantitative measure of chondrogenic differentiation and tissue development. Tissue Eng Part C Methods 16:1407–1415
Gray ML, Burstein D, Lesperance LM, Gehrke L (1995) Magnetization transfer in cartilage and its constituent macromolecules. Magn Reson Med 34:319–325
Zuo H, Yao W, Qu N, Yang S, Wang J, Cui X (2014) Quantitative evaluation in combination with nonquantitative evaluation in early patellar cartilage osteoarthritis at 3.0 T. Clin Interv Aging 9:1133–1143
Regatte RR, Akella SV, Reddy R (2005) Depth-dependent proton magnetization transfer in articular cartilage. J Magn Reson Imaging 22:318–323
Hesper T, Miese FR, Hosalkar HS et al (2015) Quantitative T2(*) assessment of knee joint cartilage after running a marathon. Eur J Radiol 84:284–289
Luke AC, Stehling C, Stahl R et al (2010) High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running: does long-distance running lead to cartilage damage? Am J Sports Med 38:2273–2280
Franciozi CE, Tarini VA, Reginato RD et al (2013) Gradual strenuous running regimen predisposes to osteoarthritis due to cartilage cell death and altered levels of glycosaminoglycans. Osteoarthritis Cartilage 21:965–972
Gee SM, Tennent DJ, Cameron KL, Posner MA (2020) The Burden of Meniscus Injury in Young and Physically Active Populations. Clin Sports Med 39:13–27
Jang H, McMillan AB, Ma Y et al (2020) Rapid single scan ramped hybrid-encoding for bicomponent T2* mapping in a human knee joint: A feasibility study. NMR Biomed 33:e4391
Pauli C, Bae WC, Lee M et al (2012) Ultrashort-echo time MR imaging of the patella with bicomponent analysis: correlation with histopathologic and polarized light microscopic findings. Radiology 264:484–493
Funding
This study has received funding by the National Natural Science Foundation of China (82101995 to Y.F.), the National Natural Science Foundation of China (82172053 to S.L.), the 2018 High-level Health Team of ZhuHai.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shaolin Li.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, D., Wu, W., Yu, W. et al. Ultrashort echo time magnetization transfer imaging of knee cartilage and meniscus after long-distance running. Eur Radiol 33, 4842–4854 (2023). https://doi.org/10.1007/s00330-023-09462-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09462-x