Abstract
Objective
To investigate the correlation of histogram metrics from diffusion-weighted imaging (DWI) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) parameters with HIF-1alpha expression in soft tissue sarcoma (STS).
Methods
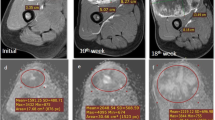
We enrolled 71 patients with STS who underwent 3.0-T MRI, including conventional MRI, DWI, and DCE-MRI sequences. Location, maximum tumor diameter, envelope, T2-weighted tumor heterogeneity, peritumoral edema, peritumoral enhancement, necrosis, tail-like pattern, bone invasion, and vessel/nerve invasion and/or encasement were determined using conventional MRI images. The whole-tumor histogram metrics were calculated on the apparent diffusion coefficient (ADC), Ktrans, Kep, and Ve maps. Independent-samples t test and one-way ANOVA were used for testing the differences between normally distributed categorical data with HIF-1alpha expression. Pearson and Spearman correlations and multiple linear regression analyses were performed to determine the correlations between histogram metrics and HIF-1alpha expression. Survival curves were plotted using the Kaplan-Meier method.
Results
Regarding conventional MRI features, only highly heterogeneous on T2-weighted images (55.6 ± 19.9% vs. 45.4 ± 20.5%, p = 0.041) and more than 50% necrotic area (57.3 ± 20.4% vs. 43.9 ± 19.7%, p = 0.002) were prone to indicate STS with higher HIF-1alpha expression. Histogram metrics obtained from ADC (mean, median, 10th, and 25th percentile values), Ktrans (mean, median, 75th, and 90th percentile values), and Kep (90th percentile values) were significantly correlated with HIF-1alpha expression. Multiple linear regression analysis demonstrated that more than 50% necrosis, ADCskewness, Ktrans90th, and grade III were independently associated with HIF-1alpha expression.
Conclusion
DWI and DCE-MRI histogram parameters were significantly correlated with HIF-1alpha expression in STS.
Key points
• DWI and DCE-MRI histogram parameters are correlated with HIF-1alpha expression in STS.
• More than 50% necrosis, ADCskewness, Ktrans90th, and grade III were independently associated with HIF-1alpha expression in STS.





Similar content being viewed by others
Abbreviations
- DCE:
-
Dynamic contrast-enhanced
- DWI:
-
Diffusion-weighted imaging
- HIF:
-
Hypoxia-inducible factor
- ICC:
-
Intraclass correlation coefficient
- MRI:
-
Magnetic resonance imaging
- ROC:
-
Receiver operating characteristic
- STS:
-
Soft tissue sarcoma
- TE:
-
Echo time
- TR:
-
Repetition time
- TIC:
-
Time-signal intensity curve
References
Clark MA, Fisher C, Judson I, Thomas JM (2005) Soft-tissue sarcomas in adults. N Engl J Med 353:701–711
Merry E, Thway K, Jones RL, Huang PH (2021) Predictive and prognostic transcriptomic biomarkers in soft tissue sarcomas. NPJ Precis Oncol 5:17
Lugano R, Ramachandran M, Dimberg A (2020) Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 77:1745–1770
Hoos A, Stojadinovic A, Mastorides S et al (2001) High Ki-67 proliferative index predicts disease specific survival in patients with high-risk soft tissue sarcomas. Cancer 92:869–874
Seo HS, Lee H, Kim S et al (2021) Paravertebral muscles as indexes of sarcopenia and sarcopenic obesity: comparison with imaging and muscle function indexes and impact on cardiovascular and metabolic disorders. AJR Am J Roentgenol 216:1596–1606
Yang JP, Liao YD, Mai DM et al (2016) Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: a meta-analysis. Angiogenesis 19:191–200
Semenza GL (2000) Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol 35:71–103
Eubank TD, Roda JM, Liu H, O'Neil T, Marsh CB (2011) Opposing roles for HIF-1alpha and HIF-2alpha in the regulation of angiogenesis by mononuclear phagocytes. Blood 117:323–332
You L, Wu W, Wang X et al (2021) The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev 41:1622–1643
Carmeliet P, Dor Y, Herbert JM et al (1998) Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394:485–490
Nystrom H, Jonsson M, Werner-Hartman L, Nilbert M, Carneiro A (2017) Hypoxia-inducible factor 1alpha predicts recurrence in high-grade soft tissue sarcoma of extremities and trunk wall. J Clin Pathol 70:879–885
Shintani K, Matsumine A, Kusuzaki K et al (2006) Expression of hypoxia-inducible factor (HIF)-1alpha as a biomarker of outcome in soft-tissue sarcomas. Virchows Arch 449:673–681
Eisinger-Mathason TS, Zhang M, Qiu Q et al (2013) Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov 3:1190–1205
Masoud GN, Li W (2015) HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 5:378–389
Yoon C, Lee HJ, Park DJ et al (2015) Hypoxia-activated chemotherapeutic TH-302 enhances the effects of VEGF-A inhibition and radiation on sarcomas. Br J Cancer 113:46–56
Liang J, Cheng Q, Huang J et al (2019) Monitoring tumour microenvironment changes during anti-angiogenesis therapy using functional MRI. Angiogenesis 22:457–470
Awasthi R, Rathore RK, Soni P et al (2012) Discriminant analysis to classify glioma grading using dynamic contrast-enhanced MRI and immunohistochemical markers. Neuroradiology 54:205–213
Li X, Yang L, Wang Q, Tao J, Pan Z, Wang S (2021) Soft tissue sarcomas: IVIM and DKI correlate with the expression of HIF-1alpha on direct comparison of MRI and pathological slices. Eur Radiol 31:4669–4679
Liu M, Guo X, Wang S et al (2013) BOLD-MRI of breast invasive ductal carcinoma: correlation of R2* value and the expression of HIF-1alpha. Eur Radiol 23:3221–3227
Yin Z, Li X, Zhang Y et al (2022) Correlations between DWI, IVIM, and HIF-1alpha expression based on MRI and pathology in a murine model of rhabdomyosarcoma. Magn Reson Med 88:871–879
Jensen RL, Mumert ML, Gillespie DL, Kinney AY, Schabel MC, Salzman KL (2014) Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro Oncol 16:280–291
Huang W, Zhang Q, Wu G et al (2021) DCE-MRI quantitative transport mapping for noninvasively detecting hypoxia inducible factor-1alpha, epidermal growth factor receptor overexpression, and Ki-67 in nasopharyngeal carcinoma patients. Radiother Oncol 164:146–154
Li X, Wu S, Li D et al (2019) Intravoxel incoherent motion combined with dynamic contrast-enhanced perfusion MRI of early cervical carcinoma: correlations between multimodal parameters and HIF-1alpha expression. J Magn Reson Imaging 50:918–929
Lindgren A, Anttila M, Rautiainen S et al (2019) Dynamic contrast-enhanced perfusion parameters in ovarian cancer: good accuracy in identifying high HIF-1alpha expression. PLoS One 14:e0221340
Xie Q, Wu J, Du Z et al (2019) DCE-MRI in human gliomas: a surrogate for assessment of invasive hypoxia marker HIF-1Alpha based on MRI-neuronavigation stereotactic biopsies. Acad Radiol 26:179–187
Borren A, Groenendaal G, van der Groep P et al (2013) Expression of hypoxia-inducible factor-1alpha and -2alpha in whole-mount prostate histology: relation with dynamic contrast-enhanced MRI and Gleason score. Oncol Rep 29:2249–2254
Huang Z, Xu X, Meng X et al (2015) Correlations between ADC values and molecular markers of Ki-67 and HIF-1alpha in hepatocellular carcinoma. Eur J Radiol 84:2464–2469
Swartz JE, Driessen JP, van Kempen PMW et al (2018) Influence of tumor and microenvironment characteristics on diffusion-weighted imaging in oropharyngeal carcinoma: a pilot study. Oral Oncol 77:9–15
Ma T, Yang S, Jing H et al (2018) Apparent diffusion coefficients in prostate cancer: correlation with molecular markers Ki-67, HIF-1alpha and VEGF. NMR Biomed 31
Choi YJ, Lee IS, Song YS, Kim JI, Choi KU, Song JW (2019) Diagnostic performance of diffusion-weighted (DWI) and dynamic contrast-enhanced (DCE) MRI for the differentiation of benign from malignant soft-tissue tumors. J Magn Reson Imaging 50:798–809
Lee JH, Yoon YC, Seo SW, Choi YL, Kim HS (2020) Soft tissue sarcoma: DWI and DCE-MRI parameters correlate with Ki-67 labeling index. Eur Radiol 30:914–924
Xiao Z, Tang Z, Zhang J et al (2020) Whole-tumor histogram analysis of monoexponential and advanced diffusion-weighted imaging for sinonasal malignant tumors: correlations with histopathologic features. J Magn Reson Imaging 51:273–285
Xie T, Zhao Q, Fu C et al (2019) Differentiation of triple-negative breast cancer from other subtypes through whole-tumor histogram analysis on multiparametric MR imaging. Eur Radiol 29:2535–2544
Khalifa F, Soliman A, El-Baz A et al (2014) Models and methods for analyzing DCE-MRI: a review. Med Phys 41:124301
Sourbron SP, Buckley DL (2013) Classic models for dynamic contrast-enhanced MRI. NMR Biomed 26:1004–1027
Zhao F, Ahlawat S, Farahani SJ et al (2014) Can MR imaging be used to predict tumor grade in soft-tissue sarcoma? Radiology 272:192–201
Crombe A, Marcellin PJ, Buy X et al (2019) Soft-tissue sarcomas: assessment of MRI features correlating with histologic grade and patient outcome. Radiology 291:710–721
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188:1622–1635
Chhabra A, Ashikyan O, Slepicka C et al (2019) Conventional MR and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: correlation with histologic grading. Eur Radiol 29:4485–4494
Kim BR, Kang Y, Lee J et al (2022) Tumor grading of soft tissue sarcomas: assessment with whole-tumor histogram analysis of apparent diffusion coefficient. Eur J Radiol 151:110319
Kim JI, Choi KU, Lee IS et al (2015) Expression of hypoxic markers and their prognostic significance in soft tissue sarcoma. Oncol Lett 9:1699–1706
Keith B, Johnson RS, Simon MC (2011) HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 12:9–22
Semenza GL (2002) HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 8:S62–S67
Funding
This study has received funding by the National Natural Science Foundation of China (No. 82171911) and National Natural Science Foundation of China for young scholars (82102013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shuang Chen, PhD.
Conflict of interest
One of the authors (Qing Li) is an employee of Siemens Healthcare. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• case-control study/diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 35 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Hu, Y., Xie, Y. et al. Whole-tumor histogram analysis of diffusion-weighted imaging and dynamic contrast-enhanced MRI for soft tissue sarcoma: correlation with HIF-1alpha expression. Eur Radiol 33, 3961–3973 (2023). https://doi.org/10.1007/s00330-022-09296-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09296-z