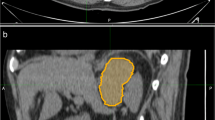
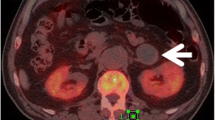
Abstract
Objectives
To investigate the effectiveness of CT-based radiomics nomograms in differentiating adrenal lipid-poor benign lesions and metastases in a cancer population.
Methods
This retrospective study enrolled 178 patients with cancer history from three medical centres categorised as those with adrenal lipid-poor benign lesions or metastases. Patients were divided into training, validation, and external testing cohorts. Radiomics features were extracted from triphasic CT images (unenhanced, arterial, and venous) to establish three single-phase models and one triphasic radiomics model using logistic regression. Unenhanced and triphasic nomograms were established by incorporating significant clinico-radiological factors and radscores. The models were evaluated by the receiver operating characteristic curve, Delong’s test, calibration curve, and decision curve.
Results
Lesion side, diameter, and enhancement ratio resulted as independent factors and were selected into nomograms. The areas under the curves (AUCs) of unenhanced and triphasic radiomics models in validation (0.878, 0.914, p = 0.381) and external testing cohorts (0.900, 0.893, p = 0.882) were similar and higher than arterial and venous models (validation: 0.842, 0.765; testing: 0.814, 0.806). Unenhanced and triphasic nomograms yielded similar AUCs in validation (0.903, 0.906, p = 0.955) and testing cohorts (0.928, 0.946, p = 0.528). The calibration curves showed good agreement and decision curves indicated satisfactory clinical benefits.
Conclusion
Unenhanced and triphasic CT-based radiomics nomograms resulted as a useful tool to differentiate adrenal lipid-poor benign lesions from metastases in a cancer population. They exhibited similar predictive efficacies, indicating that enhanced examinations could be avoided in special populations.
Key Points
• All four radiomics models and two nomograms using triphasic CT images exhibited favourable performances in three cohorts to characterise the cancer population’s adrenal benign lesions and metastases.
• Unenhanced and triphasic radiomics models had similar predictive performances, outperforming arterial and venous models.
• Unenhanced and triphasic nomograms also exhibited similar efficacies and good clinical benefits, indicating that contrast-enhanced examinations could be avoided when identifying adrenal benign lesions and metastases.






Similar content being viewed by others
Abbreviations
- APW:
-
Absolute percentage wash-out
- AUC :
-
Area under the curve
- ER:
-
Enhancement ratio
- FNAB:
-
Fine needle aspiration biopsy
- ICC:
-
Inter correlation coefficient
- LASSO:
-
Least Absolute Shrinkage and Selection Operator
- PWI:
-
Percentage wash-in
- ROC:
-
Receiver operating characteristic curve
- ROI:
-
Region of interest
- RPW:
-
Relative percentage wash-out
- RQS:
-
Radiomics quality score
References
Lam KY, Lo CY (2002) Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 56:95–101
Fassnacht M, Arlt W, Bancos I et al (2016) Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 175:G1–G34
Spartalis E, Drikos I, Ioannidis A et al (2019) Metastatic carcinomas of the adrenal glands: from diagnosis to treatment. Anticancer Res 39:2699–2710
Albano D, Agnello F, Midiri F et al (2019) Imaging features of adrenal masses. Insights Imaging 10:1
Remer EM, Obuchowski N, Ellis JD, Rice TW, Adelstein DJ, Baker ME (2000) Adrenal mass evaluation in patients with lung carcinoma: a cost-effectiveness analysis. AJR Am J Roentgenol 174:1033–1039
Elsayes KM, Emad-Eldin S, Morani AC, Jensen CT (2018) Practical approach to adrenal imaging. Urol Clin North Am 45:365–387
Caoili EM, Korobkin M, Francis IR, Cohan RH, Dunnick NR (2000) Delayed enhanced CT of lipid-poor adrenal adenomas. AJR Am J Roentgenol 175:1411–1415
Mayo-Smith WW, Song JH, Boland GL et al (2017) Management of incidental adrenal masses: a white paper of the ACR Incidental Findings Committee. J Am Coll Radiol 14:1038–1044
Mayo-Smith WW, Boland GW, Noto RB, Lee MJ (2001) State-of-the-art adrenal imaging. Radiographics 21:995–1012
Koo HJ, Choi HJ, Kim HJ, Kim SO, Cho KS (2014) The value of 15-minute delayed contrast-enhanced CT to differentiate hyperattenuating adrenal masses compared with chemical shift MR imaging. Eur Radiol 24:1410–1420
Platzek I, Sieron D, Plodeck V, Borkowetz A, Laniado M, Hoffmann RT (2019) Chemical shift imaging for evaluation of adrenal masses: a systematic review and meta-analysis. Eur Radiol 29:806–817
Boland GW, Dwamena BA, Jagtiani Sangwaiya M et al (2011) Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology. https://doi.org/10.1148/radiol.11100569/
Porte HL, Ernst OJ, Delebecq T, Métois D, Lemaitre LG, Wurtz AJ (1999) Is computed tomography guided biopsy still necessary for the diagnosis of adrenal masses in patients with resectable non-small-cell lung cancer? Eur J Cardiothorac Surg 15:597–601
Pagani JJ (1984) Non-small cell lung carcinoma adrenal metastases computed tomography and percutaneous needle biopsy in their diagnosis. Cancer 53:1058–1060
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Limkin EJ, Sun R, Dercle L et al (2017) Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol 28:1191–1206
Ji GW, Zhang YD, Zhang H et al (2019) Biliary tract cancer at CT: a radiomics-based model to predict lymph node metastasis and survival outcomes. Radiology 290:90–98
Kang B, Sun C, Gu H et al (2020) T1 stage clear cell renal cell carcinoma: A CT-based radiomics nomogram to estimate the risk of recurrence and metastasis. Front Oncol 10:579619
Chong H, Gong Y, Pan X et al (2021) Peritumoral dilation radiomics of gadoxetate disodium-enhanced MRI excellently predicts early recurrence of hepatocellular carcinoma without macrovascular invasion after hepatectomy. J Hepatocell Carcinoma 8:545–563
Wu W, Parmar C, Grossmann P et al (2016) Exploratory study to identify radiomics classifiers for lung cancer histology. Front Oncol 6:71
Stanzione A, Galatola R, Cuocolo R, Romeo V, Verde F, Mainenti PP, Brunetti A, Maurea S (2022) Radiomics in cross-sectional adrenal imaging: a systematic review and quality assessment study. Diagnostics (Basel) 12(3):578. https://doi.org/10.3390/diagnostics12030578
Shoemaker K, Hobbs BP, Bharath K, Ng CS, Baladandayuthapani V (2018) Tree-based methods for characterising tumor density heterogeneity. Pac Symp Biocomput 23:216–227
Elmohr MM, Fuentes D, Habra MA et al (2019) Machine learning-based texture analysis for differentiation of large adrenal cortical tumours on CT. Clin Radiol 74:818 e811–818 e817
Shi B, Zhang GM, Xu M, Jin ZY, Sun H (2019) Distinguishing metastases from benign adrenal masses: what can CT texture analysis do? Acta Radiol 60:1553–1561
Lee HY, Oh YL, Park SY (2021) Hyperattenuating adrenal lesions in lung cancer: biphasic CT with unenhanced and 1-min enhanced images reliably predicts benign lesions. Eur Radiol 31:5948–5958
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Mazzella A, Loi M, Mansuet-Lupo A et al (2019) Clinical characteristics, molecular phenotyping, and management of isolated adrenal metastases from lung cancer. Clin Lung Cancer 20:405–411
Moreno P, de la Quintana BA, Musholt TJ et al (2013) Adrenalectomy for solid tumor metastases: results of a multicenter European study. Surgery 154:1215–1222 discussion 1222-1213
Andersen MB, Bodtger U, Andersen IR, Thorup KS, Ganeshan B, Rasmussen F (2021) Metastases or benign adrenal lesions in patients with histopathological verification of lung cancer: Can CT texture analysis distinguish? Eur J Radiol 138:109664
Moawad AW, Ahmed A, Fuentes DT, Hazle JD, Habra MA, Elsayes KM (2021) Machine learning-based texture analysis for differentiation of radiologically indeterminate small adrenal tumors on adrenal protocol CT scans. Abdom Radiol (NY) 46:4853–4863
Ho LM, Samei E, Mazurowski MA et al (2019) Can texture analysis be used to distinguish benign from malignant adrenal nodules on unenhanced CT, contrast-enhanced CT, or In-phase and opposed-phase MRI? AJR Am J Roentgenol 212:554–561
Nagayama Y, Inoue T, Oda S et al (2020) Adrenal adenomas versus metastases: diagnostic performance of dual-energy spectral CT virtual noncontrast imaging and iodine maps. Radiology 296:324–332
Seo JM, Park BK, Park SY, Kim CK (2014) Characterization of lipid-poor adrenal adenoma: chemical-shift MRI and washout CT. AJR Am J Roentgenol 202:1043–1050
Marty M, Gaye D, Perez P et al (2018) Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol 178:439–446
Sasaguri K, Takahashi N, Takeuchi M, Carter RE, Leibovich BC, Kawashima A (2016) Differentiation of benign from metastatic adrenal masses in patients with renal cell carcinoma on contrast-enhanced CT. AJR Am J Roentgenol 207:1031–1038
Acknowledgements
We sincerely thank Jingjing Cui and Kui Sun for their assistance with radiomics analysis. We also thank Xinya Zhao for his assistance with the article revision.
Funding
This study was supported by the National Natural Science Foundation of China (81871354, 81571672) and the Academic Promotion Program of Shandong First Medical University (2019QL023).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ximing Wang.
Conflict of interest
Author Jingjing Cui declares relationships with the following companies: United Imaging Intelligence (Beijing) Co., Ltd. The remaining authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Jingjing Cui (one of the authors) and Kui Sun (not included in the authors) have significant statistical expertise.
Informed consent
The need for informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2454 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, G., Kang, B., Cui, J. et al. Two nomograms based on radiomics models using triphasic CT for differentiation of adrenal lipid-poor benign lesions and metastases in a cancer population: an exploratory study. Eur Radiol 33, 1873–1883 (2023). https://doi.org/10.1007/s00330-022-09182-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09182-8