Abstract
Objective
To compare the acute and chronic safety and treatment effects of non-invasive hepatic histotripsy vs. percutaneous microwave (MW) ablation in a healthy porcine model.
Methods
This was a dual-arm study in which each animal (n = 14) received either a single hepatic microwave (n = 6) or histotripsy (n = 6 single treatment; n = 2 double treatment) under ultrasound guidance. The goal was to create 2.5–3.0 cm short-axis treatments in similar locations across modalities. Animals were survived for 1 month with contrast-enhanced CT imaging on days 0, 2, 7, 14, and 28. On day 28, necropsy and histopathology were performed.
Results
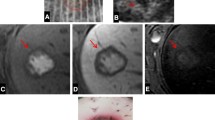
All procedures were well-tolerated. MW ablation zones were longer and more oblong, but equivalent in the short axes to histotripsy zones on immediate post-procedure CT (p < 0.001 and p = 0.45, respectively). Overall, MW volumes were larger (21.4 cm3 vs. 13.4 cm3; p = 0.001) and histotripsy treatment zones were more spherical (p = 0.007). Histotripsy zones were close to the prescribed size (p < 0.001). Over the study period, histotripsy treatment zones decreased in volume while microwave ablation zones slightly increased (−83% vs. +17%, p = 0.001). There were several imaging-only findings: Branch portal vein thrombus with both histotripsy (7/8) and MW (6/6), hematoma in 2/6 MW only, and a gallbladder injury in 1/6 MW animals. The ablation zones demonstrated complete cellular destruction for both modalities.
Conclusion
Histotripsy was associated with more spherical treatments, fewer biliary complications, and greater treatment zone involution. Hepatic MW and histotripsy treatment in a normal porcine model appear at least equally effective for creating treatment zones with a similar safety profile.
Key Points
• Microwave ablation and histotripsy for liver treatment in a healthy porcine model yield equivalent procedural tolerance and cellular destruction.
• Histotripsy was associated with more spherical treatments, fewer biliary complications, and greater treatment zone involution over the 28-day follow-up period.
• These findings confirm the safety and efficacy of hepatic histotripsy and support the pursuit of clinical trials to further evaluate the translatability of these results.






Similar content being viewed by others
Abbreviations
- CECT:
-
Contrast-enhanced computed tomography
- CK:
-
Creatine kinase
- CT:
-
Computed tomography
- FDA:
-
U.S. Food and Drug Administration
- MW:
-
Microwave
References
Benson AB, Venook AP, Al-Hawary MM et al (2021) Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 19:329–359. https://doi.org/10.6004/jnccn.2021.0012
Benson AB, D’Angelica MI, Abbott DE, et al (2021) Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 19:541–565. https://doi.org/10.6004/jnccn.2021.0022
European Association for Study of Liver, European Organisation for Research and Treatment of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer 48:599–641. https://doi.org/10.1016/j.ejca.2011.12.021
Lahat E, Eshkenazy R, Zendel A et al (2014) Complications after percutaneous ablation of liver tumors: a systematic review. Hepatobiliary Surg Nutr 3:317–323. https://doi.org/10.3978/j.issn.2304-3881.2014.09.07
Brace CL (2009) Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 38:135–143. https://doi.org/10.1067/j.cpradiol.2007.10.001
Laeseke PF, Sampson LA, Brace CL et al (2006) Unintended thermal injuries from radiofrequency ablation: protection with 5% dextrose in water. AJR Am J Roentgenol 186:S249–S254. https://doi.org/10.2214/AJR.04.1240
Livraghi T, Solbiati L, Meloni MF et al (2003) Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226:441–451. https://doi.org/10.1148/radiol.2262012198
Poon RT, Ng KK, Lam CM et al (2004) Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg 239:441–449. https://doi.org/10.1097/01.sla.0000118565.21298.0a
Parsons JE, Cain CA, Abrams GD, Fowlkes JB (2006) Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound Med Biol 32:115–129. https://doi.org/10.1016/j.ultrasmedbio.2005.09.005
Bader KB, Vlaisavljevich E, Maxwell AD (2019) For whom the bubble grows: physical principles of bubble nucleation and dynamics in histotripsy ultrasound therapy. Ultrasound Med Biol 45:1056–1080. https://doi.org/10.1016/j.ultrasmedbio.2018.10.035
Maxwell AD, Wang T-Y, Cain CA et al (2011) Cavitation clouds created by shock scattering from bubbles during histotripsy. J Acoust Soc Am 130:1888–1898. https://doi.org/10.1121/1.3625239
Xu Z, Ludomirsky A, Eun LY et al (2004) Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control 51:726–736. https://doi.org/10.1109/tuffc.2004.1308731
Maxwell AD, Cain CA, Hall TL et al (2013) Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med Biol 39:449–465. https://doi.org/10.1016/j.ultrasmedbio.2012.09.004
Vlaisavljevich E, Maxwell A, Mancia L et al (2016) Visualizing the histotripsy process: bubble cloud-cancer cell interactions in a tissue-mimicking environment. Ultrasound Med Biol 42:2466–2477. https://doi.org/10.1016/j.ultrasmedbio.2016.05.018
Vlaisavljevich E, Lin K-W, Maxwell A et al (2015) Effects of ultrasound frequency and tissue stiffness on the histotripsy intrinsic threshold for cavitation. Ultrasound Med Biol 41:1651–1667. https://doi.org/10.1016/j.ultrasmedbio.2015.01.028
Xu Z, Hall TL, Vlaisavljevich E, Lee FT (2021) Histotripsy: the first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int J Hyperth 38:561–575. https://doi.org/10.1080/02656736.2021.1905189
Smolock AR, Cristescu MM, Vlaisavljevich E et al (2018) Robotically assisted sonic therapy as a noninvasive nonthermal ablation modality: proof of concept in a porcine liver model. Radiology 287:485–493. https://doi.org/10.1148/radiol.2018171544
Longo KC, Knott EA, Watson RF et al (2019) Robotically assisted sonic therapy (RAST) for noninvasive hepatic ablation in a porcine model: mitigation of body wall damage with a modified pulse sequence. Cardiovasc Intervent Radiol 42:1016–1023. https://doi.org/10.1007/s00270-019-02215-8
Knott EA, Longo KC, Vlaisavljevich E et al (2021) Transcostal histotripsy ablation in an in vivo acute hepatic porcine model. Cardiovasc Intervent Radiol 44:1643–1650. https://doi.org/10.1007/s00270-021-02914-1
Vlaisavljevich E, Owens G, Lundt J et al (2017) Non-invasive liver ablation using histotripsy: preclinical safety study in an in vivo porcine model. Ultrasound Med Biol 43:1237–1251. https://doi.org/10.1016/j.ultrasmedbio.2017.01.016
Vlaisavljevich E, Kim Y, Owens G et al (2014) Effects of tissue mechanical properties on susceptibility to histotripsy-induced tissue damage. Phys Med Biol 59:253–270. https://doi.org/10.1088/0031-9155/59/2/253
Department of Veterinary Pathology, Iowa State University (2011) Reference Intervals. In: Iowa State University College of Veterinary Medicine. https://vetmed.iastate.edu/vpath/services/diagnostic-services/clinical-pathology/testing-and-fees/reference-intervals?field_p_type_tid=288
Ziemlewicz TJ, Hinshaw JL, Lubner MG et al (2020) Radiofrequency and microwave ablation in a porcine liver model: non-contrast CT and ultrasound radiologic-pathologic correlation. Int J Hyperth 37:799–807. https://doi.org/10.1080/02656736.2020.1784471
Knott EA, Swietlik JF, Longo KC et al (2019) Robotically-assisted sonic therapy for renal ablation in a live porcine model: initial preclinical results. J Vasc Interv Radiol 30:1293–1302. https://doi.org/10.1016/j.jvir.2019.01.023
Worlikar T, Vlaisavljevich E, Gerhardson T et al (2018) Histotripsy for non-invasive ablation of hepatocellular carcinoma (HCC) tumor in a subcutaneous xenograft murine model. Annu Int Conf IEEE Eng Med Biol Soc 2018:6064–6067. https://doi.org/10.1109/EMBC.2018.8513650
Qu S, Worlikar T, Felsted AE et al (2020) Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer 8:e000200. https://doi.org/10.1136/jitc-2019-000200
Worlikar T, Mendiratta-Lala M, Vlaisavljevich E et al (2020) Effects of histotripsy on local tumor progression in an in vivo orthotopic rodent liver tumor model. BME Front 2020:9830304. https://doi.org/10.34133/2020/9830304
Wright AS, Sampson LA, Warner TF et al (2005) Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 236:132–139. https://doi.org/10.1148/radiol.2361031249
Awad MM, Devgan L, Kamel IR et al (2007) Microwave ablation in a hepatic porcine model: correlation of CT and histopathologic findings. HPB (Oxford) 9:357–362. https://doi.org/10.1080/13651820701646222
Chiang J, Nickel K, Kimple R, Brace C (2017) Potential mechanisms of vascular thrombosis after microwave ablation in an in-vivo liver. J Vasc Interv Radiol 28:1053–1058. https://doi.org/10.1016/j.jvir.2017.03.034
Chiang J, Cristescu M, Lee MH et al (2016) Effects of microwave ablation on arterial and venous vasculature after treatment of hepatocellular carcinoma. Radiology 281:617–624. https://doi.org/10.1148/radiol.2016152508
Livraghi T, Meloni F, Solbiati L et al (2012) Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol 35:868–874. https://doi.org/10.1007/s00270-011-0241-8
Longo KC, Zlevor AM, Laeseke PF et al (2020) Histotripsy ablations in a porcine liver model: feasibility of respiratory motion compensation by alteration of the ablation zone prescription shape. Cardiovasc Intervent Radiol 43:1695–1701. https://doi.org/10.1007/s00270-020-02582-7
Denys A, Lachenal Y, Duran R et al (2014) Use of high-frequency jet ventilation for percutaneous tumor ablation. Cardiovasc Intervent Radiol 37:140–146. https://doi.org/10.1007/s00270-013-0620-4
Lubner MG, Brace CL, Hinshaw JL, Lee FT (2010) Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 21:S192–S203. https://doi.org/10.1016/j.jvir.2010.04.007
Acknowledgements
The authors would like to acknowledge the contributions of Ryan Miller, PhD; Alex Duryea, PhD; Jon Cannata, PhD; William Stoffregen, DMV, PhD; and Mike Bravo.
Funding
This study was sponsored in part by HistoSonics, Inc., Ann Arbor, MI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is TJZ.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: JLH (Consultant, Ethicon, Inc.; Shareholder, Elucent; Shareholder, Accure; Shareholder, HistoSonics, Inc.; Shareholder, Cellectar), PFL (Advisor, Shareholder, Research Support, HistoSonics, Inc.; Consultant, Ethicon, Inc.; Research Support, Siemens; Advisor, Shareholder, Elucent Medical; Shareholder, McGinley Orthopedic Innovations), CWB (Consultant, HistoSonics, Inc.), ZX (Shareholder, Consultant, Research Support, HistoSonics, Inc.), FTL (Consultant, Ethicon, Inc.; Patents, Royalties, Medtronic, Inc.; Board of Directors, Shareholder, Research Support, Histosonics, Inc.), TJZ (Shareholder, Research Support, HistoSonics, Inc.; Consultant, Research Support, Ethicon, Inc.).
Statistics and biometry
CL kindly provided statistical advice for this manuscript.
Informed consent
Approval from the institutional animal care committee was obtained.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• experimental
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Knott, E.A., Zlevor, A.M., Hinshaw, J.L. et al. A comparison study of microwave ablation vs. histotripsy for focal liver treatments in a swine model. Eur Radiol 33, 1050–1062 (2023). https://doi.org/10.1007/s00330-022-09112-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09112-8