Abstract
Objectives
Hereditary spastic paraplegia (HSP) is a group of genetic neurodegenerative diseases characterised by upper motor neuron (UMN) impairment of the lower limbs. The differential diagnosis with primary lateral sclerosis (PLS) and amyotrophic lateral sclerosis (ALS) can be challenging. As microglial iron accumulation was reported in the primary motor cortex (PMC) of ALS cases, here we assessed the radiological appearance of the PMC in a cohort of HSP patients using iron-sensitive MR imaging and compared the PMC findings among HSP, PLS, and ALS patients.
Methods
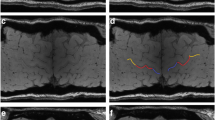
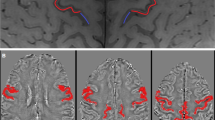
We included 3-T MRI scans of 23 HSP patients, 7 PLS patients with lower limb onset, 8 ALS patients with lower limb and prevalent UMN onset (UMN-ALS), and 84 ALS patients with any other clinical picture. The PMC was visually rated on 3D T2*-weighted images as having normal signal intensity, mild hypointensity, or marked hypointensity, and differences in the frequency distribution of signal intensity among the diseases were investigated.
Results
The marked hypointensity in the PMC was visible in 3/22 HSP patients (14%), 7/7 PLS patients (100%), 6/8 UMN-ALS patients (75%), and 35/84 ALS patients (42%). The frequency distribution of normal signal intensity, mild hypointensity, and marked hypointensity in HSP patients was different than that in PLS, UMN-ALS, and ALS patients (p < 0.01 in all cases).
Conclusions
Iron-sensitive imaging of the PMC could provide useful information in the diagnostic work - up of adult patients with a lower limb onset UMN syndrome, as the cortical hypointensity often seen in PLS and ALS cases is apparently rare in HSP patients.
Key Points
• The T2* signal intensity of the primary motor cortex was investigated in patients with HSP, PLS with lower limb onset, and ALS with lower limb and prevalent UMN onset (UMN-ALS) using a clinical 3-T MRI sequence.
• Most HSP patients had normal signal intensity in the primary motor cortex (86%); on the contrary, all the PLS and the majority of UMN-ALS patients (75%) had marked cortical hypointensity.
• The T2*-weighted imaging of the primary motor cortex could provide useful information in the differential diagnosis of sporadic adult-onset UMN syndromes.

Similar content being viewed by others
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- HSP:
-
Hereditary spastic paraplegia
- MND:
-
Motor neuron disease
- PLS:
-
Primary lateral sclerosis
- UMN:
-
Upper motor neuron
References
Shribman S, Reid E, Crosby AH, Houlden H, Warner TT (2019) Hereditary spastic paraplegia: from diagnosis to emerging therapeutic approaches. Lancet Neurol 18(12):1136–1146
Murala S, Nagarajan E, Bollu PC (2021) Hereditary spastic paraplegia. Neurol Sci 42(3):883–894
Saputra L, Kumar KR (2021) Challenges and controversies in the genetic diagnosis of hereditary spastic paraplegia. Curr Neurol Neurosci Rep 21(4):15. https://doi.org/10.1007/s11910-021-01099-x
de Souza PVS, de Rezende Pinto WBV, de Rezende Batistella GN, Bortholin T, Oliveira ASB (2017) Hereditary spastic paraplegia: clinical and genetic hallmarks. Cerebellum 16(2):525–551
Deluca GC, Ebers GC, Esiri MM (2004) The extent of axonal loss in the long tracts in hereditary spastic paraplegia. Neuropathol Appl Neurobiol 30(6):576–584
Salinas S, Proukakis C, Crosby A, Warner TT (2008) Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurol 7(12):1127–1138
Behan WM, Maia M (1974) Strümpell’s familial spastic paraplegia: genetics and neuropathology. J Neurol Neurosurg Psychiatry 37(1):8–20
Bruyn RP (1992) The neuropathology of hereditary spastic paraparesis. Clin Neurol Neurosurg 94(Suppl):S16–S18
Schwarz GA, Liu CN (1956) Hereditary (familial) spastic paraplegia; further clinical and pathologic observations. AMA Arch Neurol Psychiatry 75(2):144–162
Wakabayashi K, Kobayashi H, Kawasaki S, Kondo H, Takahashi H (2001) Autosomal recessive spastic paraplegia with hypoplastic corpus callosum, multisystem degeneration and ubiquitinated eosinophilic granules. Acta Neuropathol 101(1):69–73
Denora PS, Smets K, Zolfanelli F et al (2016) Motor neuron degeneration in spastic paraplegia 11 mimics amyotrophic lateral sclerosis lesions. Brain 139(Pt 6):1723–1734
White KD, Ince PG, Lusher M et al (2000) Clinical and pathologic findings in hereditary spastic paraparesis with spastin mutation. Neurology 55(1):89–94
Fink JK (2001) Progressive spastic paraparesis: hereditary spastic paraplegia and its relation to primary and amyotrophic lateral sclerosis. Semin Neurol 21(2):199–207
Harding AE (1981) Hereditary ‘pure’ spastic paraplegia: a clinical and genetic study of 22 families. J Neurol Neurosurg Psychiatry 44(10):871–883
Brugman F, Veldink JH, Franssen H et al (2009) Differentiation of hereditary spastic paraparesis from primary lateral sclerosis in sporadic adult-onset upper motor neuron syndromes. Arch Neurol 66(4):509–514
Fullam T, Statland J (2021) Upper motor neuron disorders: primary lateral sclerosis, upper motor neuron dominant amyotrophic lateral sclerosis, and hereditary spastic paraplegia. Brain Sci 11(5):611. https://doi.org/10.3390/brainsci11050611
Agosta F, Chiò A, Cosottini M et al (2010) The present and the future of neuroimaging in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 31(10):1769–1777
Cosottini M, Pesaresi I, Piazza S et al (2012) Structural and functional evaluation of cortical motor areas in amyotrophic lateral sclerosis. Exp Neurol 234(1):169–180
Kwan JY, Jeong SY, Van Gelderen P et al (2012) Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7(4):e35241. https://doi.org/10.1371/journal.pone.0035241
Cosottini M, Donatelli G, Costagli M et al (2016) High-resolution 7T MR imaging of the motor cortex in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 37:455–461
Costagli M, Donatelli G, Biagi L et al (2016) Magnetic susceptibility in the deep layers of the primary motor cortex in amyotrophic lateral sclerosis. Neuroimage Clin 12:965–969
Donatelli G, Retico A, Caldarazzo Ienco E et al (2018) Semiautomated evaluation of the primary motor cortex in patients with amyotrophic lateral sclerosis at 3T. AJNR Am J Neuroradiol 39(1):63–69
Donatelli G, Caldarazzo Ienco E, Costagli M et al (2019) MRI cortical feature of bulbar impairment in patients with amyotrophic lateral sclerosis. Neuroimage Clin 24:101934. https://doi.org/10.1016/j.nicl.2019.101934
Filippi M, Agosta F, Abrahams S et al (2010) EFNS guidelines on the use of neuroimaging in the management of motor neuron diseases. Eur J Neurol 17(4):526–533
Harding AE (1983) Classification of the hereditary ataxias and paraplegias. Lancet 1(8334):1151–1155
Schüle R, Holland-Letz T, Klimpe S et al (2006) The Spastic Paraplegia Rating Scale (SPRS): a reliable and valid measure of disease severity. Neurology 67(3):430–434
Turner MR, Barohn RJ, Corcia P et al (2020) Primary lateral sclerosis: consensus diagnostic criteria. J Neurol Neurosurg Psychiatry 91:373–377
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Cedarbaum JM, Stambler N, Malta E et al (1999) The ALSFRS-R: a revised ALS Functional Rating Scale that incorporates assessments of respiratory function. BDNF ALS Study Group (phase III). J Neurol Sci 169(1-2):13–21
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Hope ACA (1968) A simplified Monte Carlo significance test procedure. J R Stat Soc Series B 30:582–598
Strong MJ, Gordon PH (2005) Primary lateral sclerosis, hereditary spastic paraplegia and amyotrophic lateral sclerosis: discrete entities or spectrum? Amyotroph Lateral Scler Other Motor Neuron Disord 6(1):8–16
Gordon PH, Cheng B, Katz IB et al (2006) The natural history of primary lateral sclerosis. Neurology 66(5):647–653
Gozutok O, Helmold BR, Ozdinler PH (2021) Mutations and protein interaction landscape reveal key cellular events perturbed in upper motor neurons with HSP and PLS. Brain Sci 11(5):578. https://doi.org/10.3390/brainsci11050578
Mackay-Sim A (2021) Hereditary spastic paraplegia: from genes, cells and networks to novel pathways for drug discovery. Brain Sci 11(3):403. https://doi.org/10.3390/brainsci11030403
Fink JK (2013) Hereditary spastic paraplegia: clinico-pathologic features and emerging molecular mechanisms. Acta Neuropathol 126(3):307–328
Suzuki SO, Iwaki T, Arakawa K, Furuya H, Fujii N, Iwaki A (2011) An autopsy case of adult-onset hereditary spastic paraplegia type 2 with a novel mutation in exon 7 of the proteolipid protein 1 gene. Acta Neuropathol 122(6):775–781
Agosta F, Scarlato M, Spinelli EG et al (2015) Hereditary spastic paraplegia: beyond clinical phenotypes toward a unified pattern of central nervous system damage. Radiology 276(1):207–218
da Graça FF, de Rezende TJR, Vasconcellos LFR, Pedroso JL, Barsottini OGP, França MC Jr (2019) Neuroimaging in hereditary spastic paraplegias: current use and future perspectives. Front Neurol 9:1117. https://doi.org/10.3389/fneur.2018.01117
Lindig T, Bender B, Hauser TK et al (2015) Gray and white matter alterations in hereditary spastic paraplegia type SPG4 and clinical correlations. J Neurol 262(8):1961–1971
Ince PG, Evans J, Knopp M et al (2003) Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology 60(8):1252–1258
Philips T, Robberecht W (2011) Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol 10(3):253–263
Riad SM, Hathout H, Huang JC (2011) High T2 signal in primary lateral sclerosis supports the topographic distribution of fibers in the corpus callosum: assessing disease in the primary motor segment. AJNR Am J Neuroradiol 32(4):E61–E64
Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC (1992) Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain 115(Pt2):495–520
Tan CF, Kakita A, Piao YS et al (2003) Primary lateral sclerosis: a rare upper-motor-predominant form of amyotrophic lateral sclerosis often accompanied by frontotemporal lobar degeneration with ubiquitinated neuronal inclusions? Report of an autopsy case and a review of the literature. Acta Neuropathol 105(6):615–620
Dickson DW, Josephs KA, Amador-Ortiz C (2007) TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol 114(1):71–79
Beal MF, Richardson EP Jr (1981) Primary lateral sclerosis: a case report. Arch Neurol 38(10):630–633
Watanabe R, Iino M, Honda M, Sane J, Hara M (1997) Primary lateral sclerosis. Neuropathology 17:220–224
Paganoni S, Alshikho MJ, Zürcher NR et al (2017) Imaging of glia activation in people with primary lateral sclerosis. Neuroimage Clin 17:347–353
Mackenzie IRA (2020) Neuropathology of primary lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 21(sup1):47–51
de Bot ST, van den Elzen RTM, Mensenkamp AR et al (2010) Hereditary spastic paraplegia due to SPAST mutations in 151 Dutch patients: new clinical aspects and 27 novel mutations. J Neurol Neurosurg Psychiatry 81(10):1073–1078
Magariello A, Muglia M, Patitucci A et al (2010) Mutation analysis of the SPG4 gene in Italian patients with pure and complicated forms of spastic paraplegia. J Neurol Sci 288(1-2):96–100
D’Amore A, Tessa A, Casali C et al (2018) Next generation molecular diagnosis of hereditary spastic paraplegias: an Italian cross-sectional study. Front Neurol 9:981. https://doi.org/10.3389/fneur.2018.00981
Brugman F, Wokke JH, Scheffer H, Versteeg MH, Sistermans EA, van den Berg LH (2005) Spastin mutations in sporadic adult-onset upper motor neuron syndromes. Ann Neurol 58(6):865–869
Lee H, Deignan JL, Dorrani N et al (2014) Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA 312(18):1880–1887
DaRe JT, Vasta V, Penn J, Tran N-T B, Hahn SH (2013) Targeted exome sequencing for mitochondrial disorders reveals high genetic heterogeneity. BMC Med Genet 14:118. https://doi.org/10.1186/1471-2350-14-118
Jia X, Madireddy L, Caillier S (2018) Genome sequencing uncovers phenocopies in primary progressive multiple sclerosis. Ann Neurol 84(1):51–63
Balicza P, Varga NÁ, Bolgár B et al (2019) Comprehensive analysis of rare variants of 101 autism-linked genes in a Hungarian cohort of autism spectrum disorder patients. Front Genet 10:434. https://doi.org/10.3389/fgene.2019.00434
Mintchev N, Zamba-Papanicolaou E, Kleopa KA, Christodoulou K (2009) A novel ALS2 splice-site mutation in a Cypriot juvenile-onset primary lateral sclerosis family. Neurology 72(1):28–32
Al-Saif A, Bohlega S, Al-Mohanna F (2012) Loss of ERLIN2 function leads to juvenile primary lateral sclerosis. Ann Neurol 72(4):510–516
Acknowledgements
We thank Prof. P. Frumento for his valuable suggestions on data analysis.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Mirco Cosottini.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• observational
• multicentre study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental Figure
Examples of the scoring system used to rate the signal intensity of the primary motor cortex. Compared to the post-central cortex, the deep layers of the primary motor cortex (arrows) were rated as isointense (A), mildly hypointense (B) or marked hypointense (C) (JPG 108 kb)
Supplemental Table
Clinical and genetic data of HSP patients (DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Cosottini, M., Donatelli, G., Ricca, I. et al. Iron-sensitive MR imaging of the primary motor cortex to differentiate hereditary spastic paraplegia from other motor neuron diseases. Eur Radiol 32, 8058–8064 (2022). https://doi.org/10.1007/s00330-022-08865-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08865-6