Abstract
Objectives
This study aimed to investigate the predictability of breast MRI for pathologic complete response (pCR) by molecular subtype in patients with breast cancer receiving neoadjuvant chemotherapy (NAC) and investigate the MRI findings that can mimic residual malignancy.
Methods
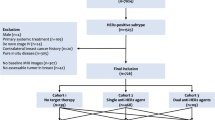
A total of 506 patients with breast cancer who underwent MRI after NAC and underwent surgery between January and December 2018 were included. Two breast radiologists dichotomized the post-NAC MRI findings as radiologic complete response (rCR) and no-rCR. The diagnostic performance of MRI predicting pCR was evaluated. pCR was determined based on the final pathology reports. Tumors were divided according to hormone receptor (HR) and human epidermal growth factor receptor (HER) 2. Residual lesions on post-NAC MRI were divided into overt and subtle which classified as nodularity or delayed enhancement. Pearson’s χ2 and Wilcoxon rank-sum tests were used for MRI findings causing false-negative pCR.
Results
The overall pCR rate was 30.04%. The overall accuracy for predicting pCR using MRI was 76.68%. The accuracy was significantly different by subtypes (p < 0.001), as follows in descending order: HR − /HER2 − (85.63%), HR + /HER2 − (82.84%), HR + /HER2 + (69.37%), and HR − /HER2 + (62.38%). MRI in the HR − /HER2 + type showed the highest false-negative rate (18.81%) for predicting pCR. The subtle residual enhancement observed only in the delayed phase was associated with false-negative findings (76.2%, p = 0.016).
Conclusions
The diagnostic accuracy of MRI for predicting pCR differed by molecular subtypes. When the residual enhancement on MRI after NAC is subtle and seen only in the delayed phase, overinterpretation of residual tumors should be performed with caution.
Key Points
• In patients with breast cancer after completion of neoadjuvant chemotherapy, the diagnostic accuracy of MRI for predicting pathologic complete response (pCR) differed according to molecular subtype.
• When residual enhancement on MRI is subtle and seen only in the delayed phase, this finding could be associated with false-negative pCR results.




Similar content being viewed by others
Abbreviations
- AC-T:
-
Adriamycin, cyclophosphamide plus docetaxel, or paclitaxel
- DCE:
-
Dynamic contrast-enhanced
- DCIS:
-
Ductal carcinoma in situ
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hormone receptor
- ICC:
-
Interclass correlation coefficient
- ĸ :
-
Cohen’s unweighted kappa
- MRI:
-
Magnetic resonance imaging
- NAC:
-
Neoadjuvant chemotherapy
- NME:
-
Non-mass enhancement
- NPV:
-
Negative predictive value
- pCR:
-
Pathologic complete response
- PPV:
-
Positive predictive value
- PR:
-
Progesterone receptor
- rCR:
-
Radiologic complete response
- SER:
-
Signal enhancement ratio
- TCHP:
-
Docetaxel, carboplatin, trastuzumab, and pertuzumab
References
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L (2001) Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 19:4224–4237
Mauri D, Pavlidis N, Ioannidis JP (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 97:188–194
Gralow JR, Burstein HJ, Wood W et al (2008) Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 26:814–819
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 26:778–785
Hennessy BT, Hortobagyi GN, Rouzier R et al (2005) Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol 23:9304–9311
Von Minckwitz G, Untch M, Blohmer J-U et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804
Dominici LS, Negron Gonzalez VM, Buzdar AU et al (2010) Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer 116:2884–2889
Boughey JC, Suman VJ, Mittendorf EA et al (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310:1455–1461
Weber JJ, Jochelson MS, Eaton A et al (2017) MRI and prediction of pathologic complete response in the breast and axilla after neoadjuvant chemotherapy for breast cancer. J Am Coll Surg 225:740–746
De Los Santos JF, Cantor A, Amos KD et al (2013) Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer: Translational Breast Cancer Research Consortium trial 017. Cancer 119:1776–1783
Cameron D, Anderson E, Levack P et al (1997) Primary systemic therapy for operable breast cancer-10-year survival data after chemotherapy and hormone therapy. Br J Cancer 76:1099–1105
Liedtke C, Mazouni C, Hess KR et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275–1281
Marinovich M, Sardanelli F, Ciatto S et al (2012) Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast 21:669–677
Loo CE, Straver ME, Rodenhuis S et al (2011) Magnetic resonance imaging response monitoring of breast cancer during neoadjuvant chemotherapy: relevance of breast cancer subtype. J Clin Oncol 29:660–666
Chen J-H, Bahri S, Mehta RS et al (2011) Breast cancer: evaluation of response to neoadjuvant chemotherapy with 3.0-T MR imaging. Radiology 261:735–743
Loo CE, Teertstra HJ, Rodenhuis S et al (2008) Dynamic contrast-enhanced MRI for prediction of breast cancer response to neoadjuvant chemotherapy: initial results. AJR Am J Roentgenol 191:1331–1338
Manton D, Chaturvedi A, Hubbard A et al (2006) Neoadjuvant chemotherapy in breast cancer: early response prediction with quantitative MR imaging and spectroscopy. Br J Cancer 94:427–435
Padhani AR, Hayes C, Assersohn L et al (2006) Prediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast-enhanced MR imaging: initial clinical results. Radiology 239:361–374
Pickles MD, Lowry M, Manton DJ, Gibbs P, Turnbull LW (2005) Role of dynamic contrast enhanced MRI in monitoring early response of locally advanced breast cancer to neoadjuvant chemotherapy. Breast Cancer Res Treat 91:1–10
Erlemann R (1993) Dynamic, gadolinium-enhanced MR imaging to monitor tumor response to chemotherapy. Radiology 186:904–905
Abraham DC, Jones RC, Jones SE et al (1996) Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer 78:91–100
Bollet MA, Thibault F, Bouillon K et al (2007) Role of dynamic magnetic resonance imaging in the evaluation of tumor response to preoperative concurrent radiochemotherapy for large breast cancers: a prospective phase II study. Int J Radiat Oncol Biol Phys 69:13–18
Lobbes M, Prevos R, Smidt M et al (2013) The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging 4:163–175
Chen JH, Bahri S, Mehta RS et al (2014) Impact of factors affecting the residual tumor size diagnosed by MRI following neoadjuvant chemotherapy in comparison to pathology. J Surg Oncol 109:158–167
Bouzon A, Acea B, Soler R et al (2016) Diagnostic accuracy of MRI to evaluate tumour response and residual tumour size after neoadjuvant chemotherapy in breast cancer patients. Radiol Oncol 50:73–79
Sutton EJ, Braunstein LZ, El-Tamer MB et al (2021) Accuracy of magnetic resonance imaging-guided biopsy to verify breast cancer pathologic complete response after neoadjuvant chemotherapy: a nonrandomized controlled trial. JAMA Netw Open 4:e2034045–e2034045
Kuerer HM, Rauch GM, Krishnamurthy S et al (2018) A clinical feasibility trial for identification of exceptional responders in whom breast cancer surgery can be eliminated following neoadjuvant systemic therapy. Ann Surg 267:946–951
Rauch GM, Kuerer HM, Adrada B et al (2018) Biopsy feasibility trial for breast cancer pathologic complete response detection after neoadjuvant chemotherapy: imaging assessment and correlation endpoints. Ann Surg Oncol 25:1953–1960
Gampenrieder SP, Peer A, Weismann C et al (2019) Radiologic complete response (rCR) in contrast-enhanced magnetic resonance imaging (CE-MRI) after neoadjuvant chemotherapy for early breast cancer predicts recurrence-free survival but not pathologic complete response (pCR). Breast Cancer Res 21:1–11
Goorts B, Dreuning KM, Houwers JB et al (2018) MRI-based response patterns during neoadjuvant chemotherapy can predict pathological (complete) response in patients with breast cancer. Breast Cancer Res 20:1–10
Kim SY, Cho N, Shin SU et al (2018) Contrast-enhanced MRI after neoadjuvant chemotherapy of breast cancer: lesion-to-background parenchymal signal enhancement ratio for discriminating pathological complete response from minimal residual tumour. Eur Radiol 28:2986–2995
Marinovich ML, Houssami N, Macaskill P et al (2013) Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Nat Cancer Inst 105:321–333
Bufi E, Belli P, Di Matteo M et al (2014) Effect of breast cancer phenotype on diagnostic performance of MRI in the prediction to response to neoadjuvant treatment. Eur J Radiol 83:1631–1638
Michishita S, Kim SJ, Shimazu K et al (2015) Prediction of pathological complete response to neoadjuvant chemotherapy by magnetic resonance imaging in breast cancer patients. Breast 24:159–165
Ogston KN, Miller ID, Payne S et al (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12:320–327
Park YJ, Youk JH, Son EJ, Gweon HM, Kim J-A (2014) Comparison of hormonal receptor and HER2 status between ultrasound-guided 14-gauge core needle biopsy and surgery in breast cancer patients. Ultrasonography 33:206–215
Fujii T, Kogawa T, Dong W et al (2017) Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann Oncol 28:2420–2428
Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Fam Med 37:360–363
Hayashi Y, Takei H, Nozu S et al (2013) Analysis of complete response by MRI following neoadjuvant chemotherapy predicts pathological tumor responses differently for molecular subtypes of breast cancer. Oncol Lett 5:83–89
Untch M, Rezai M, Loibl S et al (2010) Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol 28:2024–2031
Untch M, Fasching PA, Konecny GE et al (2011) Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol 29:3351–3357
van Ramshorst MS, Loo CE, Groen EJ et al (2017) MRI predicts pathologic complete response in HER2-positive breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 164:99–106
Ko ES, Han H, Han B-K et al (2015) Prognostic significance of a complete response on breast MRI in patients who received neoadjuvant chemotherapy according to the molecular subtype. Korean J Radiol 16:986–995
Schmitz AMT, Teixeira SC, Pengel KE et al (2017) Monitoring tumor response to neoadjuvant chemotherapy using MRI and 18F-FDG PET/CT in breast cancer subtypes. PLoS One 12:e0176782
Charehbili A, Wasser M, Smit V et al (2014) Accuracy of MRI for treatment response assessment after taxane-and anthracycline-based neoadjuvant chemotherapy in HER2-negative breast cancer. Eur J Surg Oncol 40:1216–1221
Negrão EM, Souza JA, Marques EF, Bitencourt AG (2019) Breast cancer phenotype influences MRI response evaluation after neoadjuvant chemotherapy. Eur J Radiol 120:108701
Moon H-G, Han W, Ahn SK et al (2013) Breast cancer molecular phenotype and the use of HER2-targeted agents influence the accuracy of breast MRI after neoadjuvant chemotherapy. Ann Surg 257:133–137
Navarro Vilar L, Alandete German SP, Medina Garcia R, Blanc Garcia E, Camarasa Lillo N, Vilar Samper J (2017) MR imaging findings in molecular subtypes of breast cancer according to BIRADS system. Breast J 23:421–428
Santamaria G, Bargallo X, Fernandez PL, Farrus B, Caparros X, Velasco M (2017) Neoadjuvant systemic therapy in breast cancer: association of contrast-enhanced MR imaging findings, diffusion-weighted imaging findings, and tumor subtype with tumor response. Radiology 283:663–672
Mann RM, Cho N, Moy L (2019) Breast MRI: state of the art. Radiology 292:520–536
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Boo-Kyung Han.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J., Han, BK., Ko, E.Y. et al. Prediction of pathologic complete response on MRI in patients with breast cancer receiving neoadjuvant chemotherapy according to molecular subtypes. Eur Radiol 32, 4056–4066 (2022). https://doi.org/10.1007/s00330-021-08461-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08461-0