Abstract
Objectives
To quantify hepatocellular carcinoma (HCC) and liver parenchyma stiffness using MR elastography (MRE) and serum alpha fetoprotein (AFP), before and 6 weeks (6w) after 90Y radioembolisation (RE), and to assess the value of baseline tumour and liver stiffness (TS/LS) and AFP in predicting response at 6w and 6 months (6 m).
Methods
Twenty-three patients (M/F 18/5, mean age 68.3 ± 9.3 years) scheduled to undergo RE were recruited into this prospective single-centre study. Patients underwent an MRI exam at baseline and 6w following RE (range 39–47 days) which included MRE using a prototype 2D EPI sequence. TS, peritumoural LS/LS remote from the tumour, tumour size, and AFP were measured at baseline and at 6w. Treatment response was determined using mRECIST at 6w and 6 m.
Results
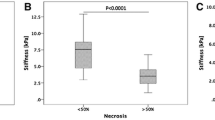
MRE was technically successful in 17 tumours which were classified at 6w as complete response (CR, n = 7), partial response (PR, n = 4), and stable disease (SD, n = 6). TS and peritumoural LS were significantly increased following RE (p = 0.016, p = 0.039, respectively), while LS remote from tumour was unchanged (p = 0.245). Baseline TS was significantly lower in patients who achieved CR at 6w (p = 0.014). Baseline TS, peritumoural LS (both AUC = 0.857), and AFP (AUC = 0.798) showed fair/excellent diagnostic performance in predicting CR at 6w, but were not significant predictors of OR or CR at 6 m.
Conclusion
Our initial results suggest that HCC TS and peritumoural LS increase early after RE. Baseline TS, peritumoural LS, and AFP were all significant predictors of CR to RE at 6w. These results should be confirmed in a larger study.
Key Points
• Magnetic resonance elastography–derived tumour stiffness and peritumoural liver stiffness increase significantly at 6 weeks post radioembolisation whereas liver stiffness remote from the tumour is unchanged.
• Baseline tumour stiffness and peritumoural liver stiffness are lower in patients who achieve complete response at 6 weeks post radioembolisation.
• Baseline tumour size is significantly correlated with baseline tumour stiffness.






Similar content being viewed by others
Abbreviations
- AFP:
-
Alpha fetoprotein
- LS:
-
Liver stiffness
- MRE:
-
Magnetic resonance elastography
- RE:
-
90Y radioembolisation
- TS:
-
Tumour stiffness
- VOI:
-
Volume of interest
References
Kulik LM, Atassi B, van Holsbeeck L et al (2006) Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 94:572–586
Lewandowski RJ, Geschwind JF, Liapi E, Salem R (2011) Transcatheter intraarterial therapies: rationale and overview. Radiology 259:641–657
Semaan S, Makkar J, Lewis S, Chatterji M, Kim E, Taouli B (2017) Imaging of hepatocellular carcinoma response after (90)Y radioembolisation. AJR Am J Roentgenol 209:W263–W276
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Gordic S, Corcuera-Solano I, Stueck A et al (2017) Evaluation of HCC response to locoregional therapy: validation of MRI-based response criteria versus explant pathology. J Hepatol 67:1213–1221
Elsayes KM, Hooker JC, Agrons MM et al (2017) 2017 Version of LI-RADS for CT and MR imaging: an update. Radiographics 37:1994–2017
Deng J, Miller FH, Rhee TK et al (2006) Diffusion-weighted MR imaging for determination of hepatocellular carcinoma response to yttrium-90 radioembolisation. J Vasc Interv Radiol 17:1195–1200
Gordic S, Wagner M, Zanato R et al (2019) Prediction of hepatocellular carcinoma response to (90)Yttrium radioembolisation using volumetric ADC histogram quantification: preliminary results. Cancer Imaging 19:29
Barker HE, Paget JT, Khan AA, Harrington KJ (2015) The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 15:409–425
Ibrahim SM, Nikolaidis P, Miller FH et al (2009) Radiologic findings following Y90 radioembolisation for primary liver malignancies. Abdom Imaging 34:566–581
Kennedy P, Wagner M, Castera L et al (2018) Quantitative elastography methods in liver disease: current evidence and future directions. Radiology 286:738–763
Chang W, Lee JM, Yoon JH et al (2016) Liver fibrosis staging with MR elastography: comparison of diagnostic performance between patients with chronic hepatitis B and those with other etiologic causes. Radiology 280:88–97
Morisaka H, Motosugi U, Glaser KJ et al (2016) Comparison of diagnostic accuracies of two- and three-dimensional MR elastography of the liver. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25425:n/a-n/a
Yin M, Glaser KJ, Talwalkar JA, Chen J, Manduca A, Ehman RL (2016) Hepatic MR elastography: clinical performance in a series of 1377 consecutive examinations. Radiology 278:114–124
Venkatesh SK, Yin M, Glockner JF et al (2008) MR elastography of liver tumors: preliminary results. AJR Am J Roentgenol 190:1534–1540
Garteiser P, Doblas S, Daire J-L et al (2012) MR elastography of liver tumours: value of viscoelastic properties for tumour characterisation. Eur Radiol 22:2169–2177
Shahryari M, Tzschatzsch H, Guo J et al (2019) Tomoelastography distinguishes noninvasively between benign and malignant liver lesions. Cancer Res 79:5704–5710
Thompson SM, Wang J, Chandan VS et al (2017) MR elastography of hepatocellular carcinoma: correlation of tumor stiffness with histopathology features-preliminary findings. Magn Reson Imaging 37:41–45
Wang J, Shan Q, Liu Y et al (2019) 3D MR elastography of hepatocellular carcinomas as a potential biomarker for predicting tumor recurrence. J Magn Reson Imaging 49:719–730
Gordic S, Ayache JB, Kennedy P et al (2017) Value of tumor stiffness measured with MR elastography for assessment of response of hepatocellular carcinoma to locoregional therapy. Abdom Radiol (NY). https://doi.org/10.1007/s00261-017-1066-y:1-10
Qayyum A, Hwang KP, Stafford J et al (2019) Immunotherapy response evaluation with magnetic resonance elastography (MRE) in advanced HCC. J Immunother Cancer 7:329
Silva AM, Grimm RC, Glaser KJ et al (2015) Magnetic resonance elastography: evaluation of new inversion algorithm and quantitative analysis method. Abdom Imaging 40:810–817
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Vogl TJ, Martin SS, Johnson AA, Haas Y (2020) Evaluation of MR elastography as a response parameter for transarterial chemoembolisation of colorectal liver metastases. Eur Radiol
Lam WA, Rosenbluth MJ, Fletcher DA (2007) Chemotherapy exposure increases leukemia cell stiffness. Blood 109:3505–3508
Raudenska M, Kratochvilova M, Vicar T et al (2019) Cisplatin enhances cell stiffness and decreases invasiveness rate in prostate cancer cells by actin accumulation. Sci Rep 9:1660
Spina JC, Hume I, Pelaez A, Peralta O, Quadrelli M, Garcia Monaco R (2019) Expected and unexpected imaging findings after (90)Y transarterial radioembolisation for liver tumors. Radiographics 39:578–595
Chen R, Kong W, Gan Y et al (2019) Tumour stiffness associated with tumour response to conventional transarterial chemoembolisation for hepatocellular carcinoma: preliminary findings. Clin Radiol 74:814.e811–814.e817
Hayashi M, Yamamoto Y, Ibusuki M et al (2012) Evaluation of tumor stiffness by elastography is predictive for pathologic complete response to neoadjuvant chemotherapy in patients with breast cancer. Ann Surg Oncol 19:3042–3049
Levental KR, Yu H, Kass L et al (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891–906
Schrader J, Gordon-Walker TT, Aucott RL et al (2011) Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 53:1192–1205
Dong Y, Zheng Q, Wang Z et al (2019) Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis. J Hematol Oncol 12:112
Finn RS, Qin S, Ikeda M et al (2020) Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894–1905
Lee MS, Ryoo BY, Hsu CH et al (2020) Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol 21:808–820
Lok AS, Sterling RK, Everhart JE et al (2010) Des-γ-carboxy prothrombin and α-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology 138:493–502
Trevisani F, D'Intino PE, Morselli-Labate AM et al (2001) Serum α-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol 34:570–575
Memon K, Kulik L, Lewandowski RJ et al (2012) Alpha-fetoprotein response correlates with EASL response and survival in solitary hepatocellular carcinoma treated with transarterial therapies: a subgroup analysis. J Hepatol 56:1112–1120
Riaz A, Ryu RK, Kulik LM et al (2009) Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol 27:5734–5742
Spreafico C, Sposito C, Vaiani M et al (2018) Development of a prognostic score to predict response to Yttrium-90 radioembolisation for hepatocellular carcinoma with portal vein invasion. J Hepatol 68:724–732
Bhutiani N, O'Brien SJ, Priddy EE et al (2020) Correlating serum alpha-fetoprotein in hepatocellular carcinoma with response to Yttrium-90 transarterial radioembolisation with glass microspheres (TheraSphere™). HPB (Oxford). https://doi.org/10.1016/j.hpb.2019.12.007
Hennedige TP, Hallinan JTPD, Leung FP et al (2016) Comparison of magnetic resonance elastography and diffusion-weighted imaging for differentiating benign and malignant liver lesions. Eur Radiol 26:398–406
Friemel J, Rechsteiner M, Frick L et al (2015) Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res 21:1951–1961
Acknowledgements
The authors thank the participants for their time and are grateful to the MR technologists for assistance with imaging.
Funding
This study was funded through NCI U01 CA172320.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Bachir Taouli.
Conflict of interest
The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
The patients reported in this study have been reported previously. There is no overlap in MR data between the studies. MR elastography data reported here has not been previously reported.
Methodology
• prospective
• Observational - longitudinal study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kennedy, P., Lewis, S., Bane, O. et al. Early effect of 90Y radioembolisation on hepatocellular carcinoma and liver parenchyma stiffness measured with MR elastography: initial experience. Eur Radiol 31, 5791–5801 (2021). https://doi.org/10.1007/s00330-020-07636-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07636-5