Abstract
Objectives
The aim of our study was to investigate whether the magnetic susceptibility varies according to the amyotrophic lateral sclerosis (ALS) phenotypes based on the predominance of upper motor neuron (UMN)/lower motor neuron (LMN) impairment.
Methods
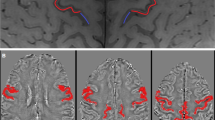
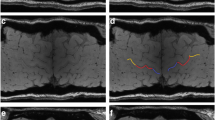
We retrospectively collected imaging and clinical data of 47 ALS patients (12 with UMN predominance (UMN-ALS), 16 with LMN predominance (LMN-ALS), and 19 with no clinically defined predominance (Np-ALS)). We further enrolled 23 healthy controls (HC) and 15 ALS mimics (ALS-Mim). These participants underwent brain 3-T magnetic resonance imaging (3-T MRI) with T1-weighted and gradient-echo multi-echo sequences. Automatic segmentation and quantitative susceptibility mapping (QSM) were performed. The skewness of the susceptibility values in the precentral cortex (SuscSKEW) was automatically computed, compared among the groups, and correlated to the clinical variables.
Results
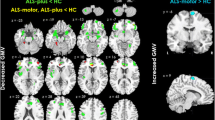
The Kruskal-Wallis test showed significant differences in terms of SuscSKEW among groups (χ2(3) = 24.2, p < 0.001), and pairwise tests showed that SuscSKEW was higher in UMN-ALS compared to those in LMN-ALS (p < 0.001), HC (p < 0.001), Np-ALS (p = 0.012), and ALS-Mim (p < 0.001). SuscSKEW was highly correlated with the Penn UMN score (Spearman’s rho 0.612, p < 0.001).
Conclusion
This study demonstrates that the clinical ALS phenotypes based on UMN/LMN sign predominance significantly differ in terms of magnetic susceptibility properties of the precentral cortex. Combined MRI-histopathology investigations are strongly encouraged to confirm whether this evidence is due to iron overload in UMN-ALS, unlike in LMN-ALS.
Key Points
• Magnetic susceptibility in the precentral cortex reflects the prevalence of UMN/LMN impairment in the clinical ALS phenotypes.
• The degree of UMN/LMN impairment might be well described by the automatically derived measure of SuscSKEW in the precentral cortex.
• Increased SuscSKEW in the precentral cortex is more relevant in UMN-ALS patients compared to those in Np-ALS and LMN-ALS patients.





Similar content being viewed by others
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- ALSFRS-R:
-
Revised Amyotrophic Lateral Sclerosis Functional Rating Scale
- ALS-Mim:
-
ALS mimics and chameleons
- C-ALS:
-
“Classic” ALS
- GRE:
-
Gradient-echo
- HC:
-
Healthy controls
- LMN:
-
Lower motor neuron
- LMN-ALS:
-
ALS patients with LMN predominance
- Np-ALS:
-
ALS patients with no clinically defined predominance
- PLS:
-
Primary lateral sclerosis
- PMA:
-
Progressive muscular atrophy
- QSM:
-
Quantitative susceptibility mapping
- SuscSKEW:
-
Skewness of the susceptibility value distribution in the precentral cortex
- UMN:
-
Upper motor neuron
- UMN-ALS:
-
ALS patients with UMN predominance
References
Ludolph A, Drory V, Hardiman O et al (2015) A revision of the El Escorial criteria - 2015. Amyotroph Lateral Scler Frontotemporal Degener 16:291–292. https://doi.org/10.3109/21678421.2015.1049183
Al-Chalabi A, Hardiman O, Kiernan MC, Chiò A, Rix-Brooks B, van den Berg LH (2016) Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol 15:1182–1194
Costagli M, Donatelli G, Biagi L et al (2016) Magnetic susceptibility in the deep layers of the primary motor cortex in amyotrophic lateral sclerosis. Neuroimage Clin 12:965–969. https://doi.org/10.1016/j.nicl.2016.04.011
Kwan JY, Jeong SY, Van Gelderen P et al (2012) Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7:e35241. https://doi.org/10.1371/journal.pone.0035241
Adachi Y, Sato N, Saito Y et al (2015) Usefulness of SWI for the detection of iron in the motor cortex in amyotrophic lateral sclerosis. J Neuroimaging 25:443–451
Contarino VE, Conte G, Morelli C et al (2020) Toward a marker of upper motor neuron impairment in amyotrophic lateral sclerosis: a fully automatic investigation of the magnetic susceptibility in the precentral cortex. Eur J Radiol 124:108815. https://doi.org/10.1016/j.ejrad.2020.108815
Schweitzer AD, Liu T, Gupta A et al (2015) Quantitative susceptibility mapping of the motor cortex in amyotrophic lateral sclerosis and primary lateral sclerosis. AJR Am J Roentgenol 204:1086–1092. https://doi.org/10.2214/AJR.14.13459
de Carvalho M, Dengler R, Eisen A et al (2008) Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 119:497–503. https://doi.org/10.1016/j.clinph.2007.09.143
Huynh W, Simon NG, Grosskreutz J, Turner MR, Vucic S, Kiernan MC (2016) Assessment of the upper motor neuron in amyotrophic lateral sclerosis. Clin Neurophysiol 127:2643–2660. https://doi.org/10.1016/j.clinph.2016.04.025
Vázquez-Costa JF, Mazón M, Carreres-Polo J et al (2018) Brain signal intensity changes as biomarkers in amyotrophic lateral sclerosis. Acta Neurol Scand 137:262–271. https://doi.org/10.1111/ane.12863
Cedarbaum JM, Stambler N, Malta E et al (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci 169:13–21. https://doi.org/10.1016/S0022-510X(99)00210-5
Turner MR, Talbot K (2013) Mimics and chameleons in motor neurone disease. Pract Neurol 13:153–164. https://doi.org/10.1136/practneurol-2013-000557
Liu C, Li W, Tong KA, Yeom KW, Kuzminski S (2015) Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging 42:23–41. https://doi.org/10.1002/jmri.24768
Fischl B (2012) FreeSurfer. Neuroimage 62:774–781. https://doi.org/10.1016/j.neuroimage.2012.01.021
Quade D (1967) Rank analysis of covariance. J Am Stat Assoc 62:1187–1200
Geser F, Stein B, Partain M et al (2011) Motor neuron disease clinically limited to the lower motor neuron is a diffuse TDP-43 proteinopathy. Acta Neuropathol 121:509–517. https://doi.org/10.1007/s00401-011-0797-z
Carrí MT, Ferri A, Cozzolino M, Calabrese L, Rotilio G (2003) Neurodegeneration in amyotrophic lateral sclerosis: the role of oxidative stress and altered homeostasis of metals. Brain Res Bull 61:365–374. https://doi.org/10.1016/s0361-9230(03)00179-5
Wang Y, Spincemaille P, Liu Z et al (2017) Clinical quantitative susceptibility mapping (QSM): biometal imaging and its emerging roles in patient care. J Magn Reson Imaging 46:951–971. https://doi.org/10.1002/jmri.25693
Sheykhansari S, Kozielski K, Bill J et al (2018) Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: a review. Cell Death Dis 9:348. https://doi.org/10.1038/s41419-018-0379-2
Ince PG, Evans J, Knopp M et al (2003) Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology 60:1252–1258. https://doi.org/10.1212/01.wnl.0000058901.75728.4e
Brownell B, Oppenheimer DR, Hughes JT (1970) The central nervous system in motor neurone disease. J Neurol Neurosurg Psychiatry 33:338–357. https://doi.org/10.1136/jnnp.33.3.338
Nishihira Y, Tan C-F, Hoshi Y et al (2009) Sporadic amyotrophic lateral sclerosis of long duration is associated with relatively mild TDP-43 pathology. Acta Neuropathol 117:45–53. https://doi.org/10.1007/s00401-008-0443-6
Riku Y, Atsuta N, Yoshida M et al (2014) Differential motor neuron involvement in progressive muscular atrophy: a comparative study with amyotrophic lateral sclerosis. BMJ Open 4:e005213. https://doi.org/10.1136/bmjopen-2014-005213
Spinelli EG, Agosta F, Ferraro PM et al (2016) Brain MR imaging in patients with lower motor neuron-predominant disease. Radiology 280:545–556. https://doi.org/10.1148/radiol.2016151846
Jeong SY, Rathore KI, Schulz K, Ponka P, Arosio P, David S (2009) Dysregulation of iron homeostasis in the CNS contributes to disease progression in a mouse model of amyotrophic lateral sclerosis. J Neurosci 29:610–619
Golko-Perez S, Amit T, Bar-Am, Youdim MBH, Weinreb O (2017) A novel iron chelator-radical scavenger ameliorates motor dysfunction and improves life span and mitochondrial biogenesis in SOD1G93A ALS mice. Neurotox Res 31:230–244
Moreau C, Danel V, Devedjian JC et al (2018) Could Conservative Iron Chelation Lead to Neuroprotection in Amyotrophic Lateral Sclerosis?© Caroline Moreau et al 2018; Published by Mary Ann Liebert, Inc. This Open Access article distributed under the terms of the Creative Commons License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Antioxid Redox Signal 29:742–748
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Giorgio Conte, Ospedale Maggiore Policlinico, Neuroradiology Unit.
Conflict of interest
Prof. Vincenzo Silani receives or has received research supports from the Italian Ministry of Health (Grant RF-201302355764), Fondazione Italiana di Ricerca per la SLA-AriSLA (Grants Exomefals and Novals), Fondazione regionale per la Ricerca Biomedica Regione Lombardia (Project nr. 2015-0023), and E-RARE JTC 2018 (Project Repetomics).
All other authors declare no disclosures of possible conflict of interest and/or commercial involvement involving contents of this manuscript.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Contarino VE, Conte G, Morelli C, et al (2020) “Toward a marker of upper motor neuron impairment in amyotrophic lateral sclerosis: a fully automatic investigation of the magnetic susceptibility in the precentral cortex.” Eur J Radiol 124:108815. https://doi.org/10.1016/j.ejrad.2020.108815
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 404 kb)
Rights and permissions
About this article
Cite this article
Conte, G., Contarino, V.E., Casale, S. et al. Amyotrophic lateral sclerosis phenotypes significantly differ in terms of magnetic susceptibility properties of the precentral cortex. Eur Radiol 31, 5272–5280 (2021). https://doi.org/10.1007/s00330-020-07547-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07547-5