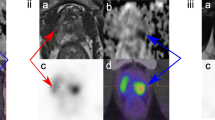
Abstract
Objective
Differentiation of malignant and benign pancreatic lesions on anatomical imaging is difficult in some cases with overlapping features. Prostate-specific membrane antigen (PSMA) is overexpressed during angioneogenesis in many tumors. We aimed to evaluate the PSMA expression in pancreatic lesions to differentiate these lesions and explore the performance of Ga-68 PSMA-PET/CT vis-a-vis F-18 FDG-PET/CT.
Methods
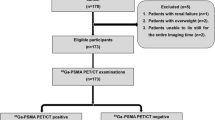
Patients with pancreatic lesions on conventional imaging were prospectively recruited. All the patients underwent a whole-body F-18 FDG-PET/CT and a regional abdominal Ga-68 PSMA-PET/CT. Focal tracer uptake (FDG or PSMA) on PET images was considered positive. Histopathology and/or cytopathology were considered the reference standard.
Results
A total of forty patients (27 males, mean age 55.3 ± 9.8, range 37–71 years) were enrolled. Of these, 19 were diagnosed as malignant on histopathology/cytology. Patients with benign lesions showed no worsening of symptoms for at least 6 months on follow-up. FDG-PET/CT revealed 17 true-positive (TP), 9 false-positive (FP), 12 true-negative (TN), and 2 false-negative (FN) findings, whereas PSMA-PET/CT had 18 TP, 2 FP, 19 TN, and 1 FN finding. The sensitivity, specificity, PPV, NPV, and accuracy for FDG-PET/CT were 89.5%, 57.1%, 65.4%, 85.7%, and 72.5%, respectively, while for PSMA-PET/CT were 94.7%, 90.5%, 90%, 95%, and 92.5%, respectively. ROC curve analysis showed that the SUVmax value of 4.8 on PSMA-PET/CT could predict the malignant potential of a lesion with a specificity of 90.5% and a sensitivity of 84.2%.
Conclusions
Ga-68 PSMA-PET/CT imaging helped in establishing a non-invasive pre-operative diagnosis of primary pancreatic malignancy with a higher degree of specificity and accuracy compared with FDG-PET/CT.
Key Points
• Conventional imaging such as CT and MRI are unable to reliably differentiate localized malignant pancreatic lesion from benign lesions mimicking malignancy such as mass-forming pancreatitis.
• FDG PET/CT helps in detecting malignant foci in view of their increased glucose metabolism. However, it may be falsely positive in inflammatory lesions which may occasionally hinder its ability to differentiate between benign and malignant lesions.
• Apart from prostatic malignancy, PSMA is overexpressed in neovasculature of many non-prostatic malignancies. The present study highlights that Ga68 PSMA PET/CT performed better in diagnosing malignancy non-invasively than FDG-PET/CT with a higher PPV (90.5% vs. 65.4%) and accuracy (92.5% vs. 72.5%).




Similar content being viewed by others
Abbreviations
- CECT:
-
Contrast-enhanced computed tomography
- EUS:
-
Endoscopic ultrasonogram
- F-18 FDG:
-
2-deoxy-2-(18F) fluoro-d-glucose
- FNAC/B:
-
Fine needle aspiration cytology/biopsy
- FWHM:
-
Full width at half maximum
- Ga-68 PSMA:
-
68Ga-Glu-NH-CO-NH-Lys(Ahx)-HBED-CC
- HBED–CC:
-
N,N′-bis [2-hydroxy-5-(carboxyethyl)benzyl] ethylenediamine N,N′-diacetic acid
- HPE:
-
Histopathological examination
- IHC:
-
Immunohistochemistry
- IPMN:
-
Intraductal papillary mucinous neoplasm
- IQR:
-
Interquartile range
- KeV:
-
Kiloelectron volt
- mA:
-
Milliampere
- MBq:
-
Megabecquerel
- mCi:
-
Millicurie
- MDCT:
-
Multidetector computed tomography
- MRCP:
-
Magnetic resonance cholangiopancreatography
- MRI:
-
Magnetic resonance imaging
- mRNA:
-
Messenger ribonucleic acid
- OSEM:
-
Ordered subset expectation maximization
- PET/CT:
-
Positron emission tomography/computed tomography
- PSMA:
-
Prostate-specific membrane antigen
- ROC:
-
Receiver operator characteristic curve
- ROI:
-
Region of interest
- SUVmax:
-
Standardized uptake value
- SUVmaxFDG :
-
Standardized uptake value – maximum on 18F-FDG PET/CT
- SUVmaxPSMA :
-
Standardized uptake value – maximum on 68Ga-PSMA PET/CT
- TBR:
-
Target to background ratio
- TP/TN/FP/FN:
-
True positive/true negative/false positive/false negative
- USG:
-
Ultrasonography
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012: Globocan 2012. Int J Cancer 136:E359–E386
Hidalgo M, Cascinu S, Kleeff J et al (2015) Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology. 15:8–18
Nichols MT, Russ PD, Chen YK (2006) Pancreatic imaging: current and emerging technologies. Pancreas. 33:211–220
Eloubeidi MA, Desmond RA, Wilcox CM et al (2006) Prognostic factors for survival in pancreatic cancer: a population-based study. Am J Surg 192:322–329
Oto A, Eltorky MA, Dave A et al (2006) Mimicks of pancreatic malignancy in patients with chronic pancreatitis: correlation of computed tomography imaging features with histopathologic findings. Curr Probl Diagn Radiol 35:199–205
Taylor B (2003) Carcinoma of the head of the pancreas versus chronic pancreatitis: diagnostic dilemma with significant consequences. World J Surg 27:1249–1257
Friess H, Langhans J, Ebert M et al (1995) Diagnosis of pancreatic cancer by 2 [18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Gut. 36:771–777
Matsumoto I, Shirakawa S, Shinzeki M et al (2013) 18-Fluorodeoxyglucose positron emission tomography does not aid in diagnosis of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 11:712–718
Maurer T, Eiber M, Schwaiger M, Gschwend JE (2016) Current use of PSMA–PET in prostate cancer management. Nat Rev Urol 13:226–235
Bouchelouche K, Choyke PL, Capala J (2010) Prostate specific membrane antigen—a target for imaging and therapy with radionuclides. Discov Med 9:55–61
Ren H, Zhang H, Wang X, Liu J, Yuan Z, Hao J (2014) Prostate-specific membrane antigen as a marker of pancreatic cancer cells. Med Oncol. https://doi.org/10.1007/s12032-014-0857-z
Tummala P, Junaidi O, Agarwal B (2011) Imaging of pancreatic cancer: an overview. J Gastrointest Oncol 2:168–174
Lee ES, Lee JM (2014) Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol 20:7864–7877
Ahn SS, Kim M-J, Choi J-Y, Hong H-S, Chung YE, Lim JS (2009) Indicative findings of pancreatic cancer in prediagnostic CT. Eur Radiol 19:2448–2455
Gallamini A, Zwarthoed C, Borra A (2014) Positron emission tomography (PET) in oncology. Cancers (Basel) 6:1821–1889
Wang Z, Chen JQ, Liu JL, Qin XG, Huang Y (2013) FDG-PET in diagnosis, staging and prognosis of pancreatic carcinoma: a meta-analysis. World J Gastroenterol 19:4808–4817
Kauhanen SP, Komar G, Seppänen MP et al (2009) A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 250:957–963
Santhosh S, Mittal BR, Bhasin D et al (2013) Role of 18F-fluorodeoxyglucose positron emission tomography /computed tomography in the characterization of pancreatic masses: experience from tropics: fluorodeoxyglucose in pancreatic masses. J Gastroenterol Hepatol 28:255–261
Jha P, Bijan B (2015) PET/CT for pancreatic malignancy: potential and pitfalls. J Nucl Med Technol 43:92–97
Heinrich S, Goerres GW, Schäfer M et al (2005) Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 242:235–243
Kato K, Nihashi T, Ikeda M et al (2013) Limited efficacy of 18F-FDG PET/CT for differentiation between metastasis-free pancreatic cancer and mass-forming pancreatitis. Clin Nucl Med 38:417–421
Ghaneh P, Hanson R, Titman A et al (2018) PET-PANC: multicentre prospective diagnostic accuracy and health economic analysis study of the impact of combined modality 18fluorine-2-fluoro-2-deoxy-d-glucose positron emission tomography with computed tomography scanning in the diagnosis and management of pancreatic cancer. Health Technol Assess 22:1–114
Fendler WP, Eiber M, Beheshti M et al (2017) 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging 44:1014–1024
Eder M, Schäfer M, Bauder-Wüst U et al (2012) 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem 23:688–697
Malik D, Kumar R, Mittal BR, Singh H, Bhattacharya A, Singh SK (2018) 68Ga-labeled PSMA uptake in nonprostatic malignancies: has the time come to remove “PS” from PSMA? Clin Nucl Med 43:529–532
Malik D, Sood A, Mittal BR et al (2018) Nonspecific uptake of 68Ga-prostate-specific membrane antigen in diseases other than prostate malignancy on positron emission tomography/computed tomography imaging: a pictorial assay and review of literature. Indian J Nucl Med 33:317–325
Krishnaraju VS, Basher RK, Singh H, Singh SK, Bal A, Mittal BR (2018) Incidental detection of type B2 thymoma on 68Ga-labeled prostate-specific membrane antigen PET/CT imaging. Clin Nucl Med 43:356–358
Malik D, Kumar R, Mittal BR et al (2018) 68Ga-labelled PSMA (prostate specific membrane antigen) expression in signet-ring cell gastric carcinoma. Eur J Nucl Med Mol Imaging 45:1276–1277
Sasikumar A, Joy A, Nanabala R, Pillai MRA, Thomas B, Vikraman KR (2016) (68)Ga-PSMA PET/CT imaging in primary hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 43:795–796
Malik D, Basher RK, Mittal BR, Jain TK, Bal A, Singh SK (2017) 68Ga-PSMA expression in pseudoangiomatous stromal hyperplasia of the breast. Clin Nucl Med 42:58–60
Jain TK, Jois AGS, Kumar VS, Singh SK, Kumar R, Mittal BR (2017) Incidental detection of tracer avidity in meningioma in 68Ga-PSMA PET/CT during initial staging for prostate cancer. Rev Esp Med Nucl Imagen Mol 36:133–134
Sasikumar A, Joy A, Pillai MRA, Alex TM, Narayanan G (2017) 68Ga-PSMA PET/CT in osteosarcoma in fibrous dysplasia. Clin Nucl Med 42:446–447
Vamadevan S, Shetty D, Le K, Bui C, Mansberg R, Loh H (2016) Prostate-specific membrane antigen (PSMA) avid pancreatic neuroendocrine tumor. Clin Nucl Med 41:804–806
Sahbai S, Rieping P, Pfannenberg C, la Fougére C, Reimold M (2017) Pancreatic ductal adenocarcinoma with high radiotracer uptake in 68Ga–prostate-specific membrane antigen PET/CT. Clin Nucl Med 42:717–718
Stock K, Steinestel K, Wiesch R et al (2017) Neovascular prostate-specific membrane antigen expression is associated with improved overall survival under palliative chemotherapy in patients with pancreatic ductal adenocarcinoma. Biomed Res Int. https://doi.org/10.1155/2017/2847303
Conway RE, Petrovic N, Li Z, Heston W, Wu D, Shapiro LH (2006) Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol 26:5310–5324
Chang SS, O’Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB (1999) Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res 5:2674–2681
Chan M, Schembri GP, Hsiao E (2017) Serous cystadenoma of the pancreas showing uptake on 68Ga PSMA PET/CT. Clin Nucl Med 42:56–57
Pyka T, Weirich G, Einspieler I et al (2016) 68Ga-PSMA-HBED-CC PET for differential diagnosis of suggestive lung lesions in patients with prostate cancer. J Nucl Med 57:367–371
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Bhagwant Rai Mittal.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Venkata Subramanian Krishnaraju, who is one of the authors, has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krishnaraju, V.S., Kumar, R., Mittal, B.R. et al. Differentiating benign and malignant pancreatic masses: Ga-68 PSMA PET/CT as a new diagnostic avenue. Eur Radiol 31, 2199–2208 (2021). https://doi.org/10.1007/s00330-020-07318-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07318-2