Abstract
Objectives
To compare the pulmonary chest CT findings of patients with COVID-19 pneumonia with those with other types of viral pneumonia.
Methods
This retrospective review includes 154 patients with RT-PCR-confirmed COVID-19 pneumonia diagnosed between February 11 and 20, 2020, and 100 patients with other types of viral pneumonia diagnosed between April 2011 and December 2020 from two hospitals. High-resolution CT (HRCT) of the chest was performed. Data on location, distribution, attenuation, maximum lesion range, lobe involvement, number of lesions, air bronchogram signs, Hilar and mediastinal lymph node enlargement, and pleural effusion were collected. Associations between imaging characteristics and COVID-19 pneumonia were analyzed with univariate and multivariate logistic regression models.
Results
A peripheral distribution was associated with a 13.04-fold risk of COVID-19 pneumonia, compared with a diffuse distribution. A maximum lesion range > 10 cm was associated with a 9.75-fold risk of COVID-19 pneumonia, compared with a maximum lesion range ≤ 5 cm, and the involvement of 5 lobes was associated with an 8.45-fold risk of COVID-19 pneumonia, compared with a maximum lesion range ≤ 2. No pleural effusion was associated with a 3.58-fold risk of COVID-19 pneumonia compared with the presence of pleural effusion. Hilar and mediastinal lymph node enlargement was associated with a 2.79-fold risk of COVID-19 pneumonia.
Conclusion
A peripheral distribution, a lesion range > 10 cm, involvement of 5 lobes, presence of hilar and mediastinal lymph node enlargement, and no pleural effusion were significantly associated with 2019-novel coronavirus pneumonia.
Key Points
• A peripheral distribution, a lesion range > 10 cm, involvement of 5 lobes, presence of hilar and mediastinal lymph node enlargement, and no pleural effusion were significantly associated with COVID-19 compared with other types of viral pneumonia.
Similar content being viewed by others
Introduction
Since December 2019, a succession of cases of pneumonia with unknown causes has been observed in Wuhan, Hubei Province, China. On January 7, 2020, the 2019 novel coronavirus (SARS-Cov2, with the disease now officially named COVID-19 by the World Health Organization) was identified as the causative agent, based on virus typing [1, 2]. Recent studies revealed that SARS-Cov 2 is related to bat-SL-CoV ZC45 and bat-SL-CoV ZXC21 [2] and can spread from human to human, mainly through respiratory droplets, physical contact, and the oral-fecal route [3].
The confirmed diagnosis of COVID-19 pneumonia requires viral nucleic acid detection in throat swabs, sputum, lower respiratory tract secretions, or blood; while the specificity of this test is strong, its sensitivity is poor [4] and most patients show multiple negative results. Furthermore, in most patients, lung imaging findings are observed earlier than clinical symptoms, which make imaging examinations crucial for screenings and an accurate diagnosis [5]. Chest CT is now the routine for patients with COVID-19 pneumonia at many institutions. In our experience, some patients with positive CT findings may have initial negative result before they were finally confirmed as COVID-19 pneumonia, and they were easily misdiagnosed as common types of viral pneumonia, especially in the non-epidemic area. In addition, there have been a few reports about COVID-19 pneumonia findings on chest CT [5,6,7,8,9,10,11]; however, the reported CT findings of COVID-19 pneumonia were extremely similar to common types of viral pneumonia. To the best of our knowledge, no study so far has compared CT findings between COVID-19 pneumonia and other types of viral pneumonia. Hence, the primary objective of our study was to compare the pulmonary CT findings of patients with COVID-19 pneumonia with those with other types of viral pneumonia.
Materials and methods
Patients
This retrospective cross-sectional study was reviewed and approved by the Biomedical Research Ethics Committee of our institution, and patient consent was waived.
All patients with a history of infectious pneumonia from April 2011 to February 2020 at Changhai Hospital and all patients with a clinical suspicion of COVID-19 from February 11 to February 20 at Huoshenshan Hospital were included. The inclusion criteria were (1) the availability of a positive reverse-transcription polymerase chain reaction (RT-PCR) tests confirming the viral origin of pneumonia and (2) the availability of a chest CT at the time of diagnosis. The exclusion criteria were (1) laboratory findings in favor of a bacterial origin, (2) normal lung parenchyma on chest CT, (3) presence of non-infectious lung parenchyma lesions on chest CT (e.g., lung cancer, pneumothorax, pulmonary edema), and (4) a delay between chest CT and RT-PCR longer than 7 days. All clinical results were extracted from the patients’ electronic medical records in the two-hospital information system. All patients with COVID-19 pneumonia were divided into four clinical types namely mild, moderate, severe, and critical [12].
CT scanning
Pulmonary CT was performed using 64-, 256-, and 128-slice multidetector row CT scanners (64: Somatom, Siemens Healthcare; 256: Brilliance-16P, Philips Healthcare; 128: uCT 760, United Imaging Healthcare). Images were captured at window settings that allowed viewing of the lung parenchyma (window level, − 600 to – 700 HU; window width, 1200–1500 HU) and the mediastinum (window level, 20–40 HU; window width, 400 HU). The scanning range covered the area from the level of the superior aperture of the thorax to the diaphragm. The CT acquisition parameters are reported in Supplemental Digital Content 1.
Radiological imaging analysis
We used the original cross-sectional images for analysis. All images were analyzed by two chest radiologists (X.L. and X.F., both with 8 years of experience) who were blinded to the clinical details. When their readings were not consistent, the final results were determined by consensus.
All lesions were evaluated for the following characteristics: (a) location: right, left, or bilateral lungs; (b) distribution: peripheral, central, or diffuse; (c) attenuation: ground glass attenuation including ground glass opacity (GGO) and crazy-paving pattern, consolidation, and mixed patterns of ground glass attenuation and consolidation [13, 14]; (d) maximum lesion range: ≤ 5 cm, 5–10 cm, and > 10 cm only for the biggest one; (e) lobe involvement: the five lung lobes were divided into categories of ≤ 2 lobes, 2–4 lobes, and = 5 lobes; (f) number of lesions: one, two, and three or more; (g) air bronchogram; (h) Hilar and mediastinal lymph nodes enlargement: short-axis diameter of a lymph node > 10 mm [15]; and (i) pleural effusion.
Statistical analyses
Normal distribution and variance homogeneity tests were performed on all continuous variables; those with a normal distribution are expressed as the mean and standard deviation while those with non-normal distributions are expressed as medians and ranges. In this study, we divided the patients into two groups (COVID-19 pneumonia and the other viral pneumonia). First, we examined group differences in all variables between patients with COVID-19 pneumonia and patients with other types of viral pneumonia. The Kruskal-Wallis H test (skewed distribution) and chi-square tests (categorical variables) were used to determine statistical differences between the two groups. Second, a univariate regression analysis was applied to estimate effect sizes for the relationships between all variables and two types of viral pneumonia. Last, multivariable logistic models were used to evaluate the associations between exposure (imaging characteristics) and outcome (viral pneumonia). These models included model 1 (not adjusted for other co-variants), model 2 (adjusted for age, sex, and body mass index [BMI]), and model 3 (adjusted for the same factors as model 2 as well as for other significantly associated clinical and imaging characteristics in univariate regression analysis). The group with other types of viral pneumonia was considered the reference group.
A two-tailed p value of less than 0.05 was considered statistically significant. All analyses were performed using R software (version 3.3.3; The R Foundation for Statistical Computing) and EmpowerStats (X&Y Solutions, Inc).
Results
Clinical characteristics
We retrospectively analyzed 254 patients at two different hospitals in China. A total of 147 consecutive patients with COVID-19 pneumonia, 71 males (mean age, 53.17 years; age range, 26–88 years) and 76 females (mean age, 58.22 years; age range, 22–83 years), were included at Huoshenshan Hospital between February 11 and 20, 2020. Seven consecutive patients with COVID-19 pneumonia, 5 males (mean age, 47.80 years; age range, 28–75 years) and 2 females (mean age, 59 years; age range, 47–71 years), were included at Changhai Hospital between January 25 and February 9, 2020. One hundred consecutive adult patients with other types of viral pneumonia, 55 males (mean age, 52.98 years; age range, 20–91 years) and 45 females (mean age, 51.13 years; age range, 20–95 years), were included at Changhai Hospital between April 2011 and December 2020. The patients with COVID-19 pneumonia and the other types of viral pneumonia were confirmed by viral nucleic acid detection (Fig. 1). The 154 patients with COVID-19 pneumonia included 114 moderate cases (74.03%) and 40 severe cases (25.97%). The 100 patients with other types of viral pneumonia included 40 cases (40.0%) of Epstein-Barr virus infections, 29 cases (29.0%) of cytomegalovirus infections, three cases (3.0%) of adenovirus infections, and 28 cases (28.0%) of influenza infections. The interval from the onset of symptoms to the positive CT findings was 8 days (range 4–13 days) and 8 days (range 3–15 days) in COVID-19 pneumonia group and the other viral pneumonia group, respectively. Among the clinical and imaging characteristics that we investigated, we found significant differences in smoking history, presence of cough, lymphocyte ratios, C-reactive protein (CRP) levels, distribution, maximum lesion range, lobe involvement, hilar and mediastinal lymph node enlargement, and pleural effusion between patients with COVID-19 pneumonia and those with other types of viral pneumonia (p > 0.05). The patient characteristics are shown in Tables 1 and 2. The patient CT findings are shown in Figs. 2 and 3.
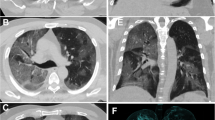
Unenhanced CT images from patients with COVID-19 pneumonia. a, b A 59-year-old woman. a CT image of the mediastinum showing a mediastinal enlarged lymph node. b CT image of lung parenchyma showing multi-focal crazy-paving pattern and consolidation. c, d A 70-year-old woman. c CT image of the mediastinum showing a mediastinal and right hilar enlarged lymph node. d CT image of lung parenchyma showing multi-focal GGO, crazy-paving pattern, and consolidation. GGO, ground glass opacity
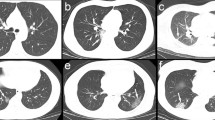
Unenhanced CT images of lung parenchyma from patients with other types of viral pneumonia. a CT image of a 23-year-old woman with influenza infection showing consolidation in the right middle lobe and GGO in the left inferior lobar. b CT image of a 64-year-old man with Epstein-Barr virus infection showing a mixed pattern of GGO and consolidation in the right middle lobe. c CT image of a 20-year-old man with adenovirus infection showing consolidation in the left inferior lobe. d CT image of a 24-year-old man with cytomegalovirus infection showing multi-focal GGO and consolidation. GGO: ground glass opacity
Univariate analysis
The results of our univariate analysis are shown in Table 3. Smoking history, cough, lymphocyte, and CRP, distribution, maximum lesion range, involvement of lobes, hilar and mediastinal lymph node enlargement, and pleural effusion were significantly associated with COVID-19 pneumonia.
Multivariate analyses
When applying the crude model (model 1), peripheral distribution, maximum lesion range > 10 cm, involvement of 5 lobes, presence of hilar and mediastinal lymph node enlargement, and no pleural effusion were significantly associated with COVID-19 pneumonia. Furthermore, in the minimally adjusted model 2 and the fully adjusted model 3, effect sizes also showed significant correlations with viral pneumonia. The results of the multivariate analysis are shown in Table 4.
Discussion
The present study aimed to compare the pulmonary CT findings of patients with 2019 novel coronavirus pneumonia with those with other types of viral pneumonia. Our analyses, in particular our fully adjusted model (model 3), show that peripheral distribution, a lesion range > 10 cm, involvement of 5 lobes, presence of hilar and mediastinal lymph node enlargement, and no pleural effusion were significantly associated with 2019 novel coronavirus pneumonia.
Our study shows the following imaging characteristics of COVID-19 pneumonia: The most common location was the bilateral lungs (91.56%) and the most common distribution was diffuse (68.18%). The most common attenuation was a mixed pattern of GGO and consolidation (72.08%). The vast majority of patients with COVID-19 pneumonia showed a large lesion range (5–10 cm, 48.05%; > 10 cm, 31.17%), ≥3 lesions (98.05%), and the involvement of five lobes (77.92%). Pan et al [6] assessed the imaging findings of 63 patients with COVID-19 pneumonia and found that 7 (11.1%) patients had four affected lobes, 28 (44.4%) patients had five affected lobes, and 54 (85.7%) patients showed patchy/punctate GGO. Pan et al [6] and Xie et al [7] both reported GGO and/or mixed GGO and mixed consolidation as imaging characteristics of COVID-19 pneumonia. In addition, some earlier studies assessed CT findings of COVID-19 pneumonia, including GGO, and reported crazy-paving patterns, consolidation, the involvement of multiple lobes, and a diffuse distribution [8,9,10,11, 16,17,18]. The current results are thus consistent with the findings of previous studies.
The present study does also provide some new findings. Firstly, we found that a peripheral distribution was associated with a 13.04-fold risk of COVID-19 pneumonia, compared with a diffuse distribution. Furthermore, a maximum lesion range > 10 cm was associated with a 9.75-fold risk of COVID-19 pneumonia, compared with a maximum lesion range ≤ 5 cm, and the involvement of 5 lobes was associated with an 8.45-fold risk of COVID-19 pneumonia, compared with the involvements of lobes ≤ 2. These findings show that the larger area and more lobes were involved in patients with COVID-19 pneumonia than in those with other types of viral pneumonia, but the distribution was peripheral, which was different from the diffuse distribution of the other vital pneumonia. Secondly, no pleural effusion was associated with a 3.58-fold risk of COVID-19 pneumonia compared with the presence of pleural effusion. This finding was associated with few alveolar exudations of interstitial pneumonitis. Notably, hilar and mediastinal lymph node enlargement was found in a few patients and was observed in 43.51% and 20.0% of patients with COVID-19 pneumonia and other types of viral pneumonia, respectively. However, hilar and mediastinal lymph node enlargement was associated with a 2.79-fold risk of COVID-19 pneumonia. This result was different from the study by Bernheim et al [19]. The following reasons would lead to the difference. First, we only included the positive CT finding; however, Bernheim’s study included 20/35 (56%) negative CT findings. Second, the interval from the onset of symptoms to the positive CT findings in this study was different from that in Bernheim’s study. We speculate that hilar and mediastinal lymph node enlargement is associated with immune responses and that patients with COVID-19 pneumonia have stronger immune responses than those with other types of vital pneumonia, especially in the moderate and severe patients with COVID-19 pneumonia.
Our study has several limitations. Firstly, this is a retrospective study with a limited number of patients in both groups. Secondly, all patients with a negative chest CT were excluded, as well as patients with other non-infectious chest CT findings, which could lead to potential inclusion bias and weaken our findings. Thirdly, there may have been a supplementary selection bias in these institutions in terms of which patients were imaged with CT. For instance, patients with moderate and severe COVID-19 pneumonia usually underwent a CT scan, while mild or asymptomatic patients do not. Fourthly, some patients may have received medical intervention once suspected or confirmed to have infection, which was not accounted for in this work. Finally, the group with other types of viral pneumonia only included common viruses and might not be a significant representation of all viral infections.
To summarize, a peripheral distribution, a lesion range > 10 cm, involvement of 5 lobes, presence of hilar and mediastinal lymph node enlargement, and no pleural effusion were significantly associated with the 2019 novel coronavirus pneumonia.
Abbreviations
- BMI:
-
Body mass index
- COVID-19:
-
2019 novel coronavirus
- CRP:
-
C-reactive protein
- GGO:
-
Ground glass opacity
- HRCT:
-
High-resolution CT
- RT-PCR:
-
Reverse-transcription polymerase chain reaction
References
Zhu N, Zhang D, Wang W et al (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733
Chen Y, Liu Q, Guo D (2020) Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 92:418–423
Chan JF, Yuan S, Kok KH et al (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395:514–523
Tan W, Zhao X, Ma X et al (2020) A novel coronavirus genome identified in a cluster of pneumonia cases- Wuhan, China 2019−2020. China CDC Weekly 2:61–62
Pan Y, Guan H, Zhou S et al (2020) Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. https://doi.org/10.1007/s00330-020-06731-x
Pan F, Ye T, Sun P et al (2020) Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. https://doi.org/10.1148/radiol.2020200370:200370
Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J (2020) Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. https://doi.org/10.1148/radiol.2020200343
Shi H, Han X, Zheng C (2020) Evolution of CT manifestations in a patient recovered from 2019 novel coronavirus (2019-nCoV) pneumonia in Wuhan, China. Radiology 295:20
Lei J, Li J, Li X, Qi X (2020) CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295:18
Fang Y, Zhang H, Xu Y, Xie J, Pang P, Ji W (2020) CT manifestations of two cases of 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295:208–209
Duan YN, Qin J (2020) Pre- and posttreatment chest CT findings: 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295:21
General Office of National Health Committee. Office of State Administration of Traditional Chinese Medicine. Notice on the issuance of a program for the diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (trial seventh edition) (2020-03-4). http://bgs.satcm.gov.cn/zhengcewenjian/2020-03-04/13594.html
Franquet T (2011) Imaging of pulmonary viral pneumonia. Radiology 260:18–39
Koo HJ, Lim S, Choe J, Choi SH, Sung H, Do KH (2018) Radiographic and CT features of viral pneumonia. Radiographics 38:719–739
Harisinghani MG (2013) Atlas of lymph node anatomy. Springer, New York
Liu P, Tan XZ (2020) 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295:19
Xu X, Yu C, Qu J et al (2020) Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging 47:1275–1280
Lin X, Gong Z, Xiao Z, Xiong J, Fan B, Liu J (2020) Novel coronavirus pneumonia outbreak in 2019: computed tomographic findings in two cases. Korean J Radiol 21:365–368
Bernheim A, Mei X, Huang M et al (2020) Chest CT findings in coronavirus Disease-19 (COVID-19): relationship to duration of infection. Radiology. https://doi.org/10.1148/radiol.2020:200463
Funding
This work was supported in part by the National Science Foundation for Scientists of China (81871352) and the National Science Foundation for Young Scientists of China (81701689).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yun Bian.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained by the Changhai hospital and Huoshenshan Hospital.
Methodology
-
Retrospective
-
Case-control study
-
Performed at a multicenter institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiao Li and Xu Fang are the joint first authors in the paper.
Electronic supplementary material
ESM 1
(DOCX 12.7 kb)
Rights and permissions
About this article
Cite this article
Li, X., Fang, X., Bian, Y. et al. Comparison of chest CT findings between COVID-19 pneumonia and other types of viral pneumonia: a two-center retrospective study. Eur Radiol 30, 5470–5478 (2020). https://doi.org/10.1007/s00330-020-06925-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06925-3