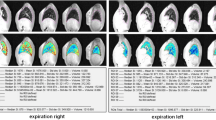
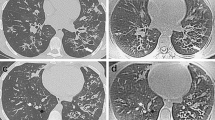
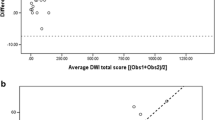
Abstract
Objectives
The study aimed to validate automated quantification of high and low signal intensity volumes using ultrashort echo-time MRI, with CT and pulmonary function test (PFT) as references, to assess the severity of structural alterations in cystic fibrosis (CF).
Methods
This prospective study was performed in a single center between May 2015 and September 2017. Participants with CF completed clinical examination, CT, MRI, and PFT the same day during routine clinical follow-up (M0), and then 1 year after (M12) except for CT. Using MRI, percentage high (%MR-HSV), low (%MR-LSV), and total abnormal (%MR-TSV) signal intensity volumes were recorded, as well as their corresponding attenuation values using CT (%CT-HAV, %CT-LAV, %CT-TAV, respectively). Automated quantifications and visual Bhalla score were evaluated independently by two observers. Correlations were assessed using the Spearman test, comparisons using the Mann-Whitney test, and reproducibility using the intraclass correlation coefficient (ICC).
Results
A total of 30 participants were enrolled (median age 27 years, 18 men). At M0, there was a good correlation between %MR-HSV and %CT-HAV (ρ = 0.70; p < 0.001) and %MR-LSV and %CT-LAV (ρ = 0.60; p < 0.001). Automated MR metrics correlated to PFTs and Bhalla score (p < 0.05) while %MR-TSV was significantly different between CF with and without respiratory exacerbation (p = 0.01) at both M0 and M12. The variation of %MR-HSV correlated to the variation of FEV1% at PFT (ρ = − 0.49; p = 0.008). Reproducibility was almost perfect (ICCs > 0.95).
Conclusions
Automated quantification of abnormal signal intensity volumes relates to CF severity and allows reproducible cross-sectional and longitudinal assessment.
Trial registration
Clinical trial identifier: NCT02449785
Key Points
• Cross-sectionally, the automated quantifications of high and low signal intensity volumes at UTE correlated to the quantification of high and low attenuation using CT as reference.
• Longitudinally, the variation of high signal intensity volume at UTE correlated to the variation of pulmonary function test and was significantly reduced in CF with an improvement in exacerbation status.
• Automated quantification of abnormal signal intensity volumes are objective and reproducible tools to assess structural alterations in CF and follow-up longitudinally, for both research and clinical purposes.





Similar content being viewed by others
Abbreviations
- %CT-HAV:
-
Percentage high attenuation volume
- %CT-LAV:
-
Percentage low attenuation volume
- %CT-TAV:
-
Percentage total abnormal attenuation volume
- %MR-HSV:
-
Percentage high signal intensity volume
- %MR-LSV:
-
Percentage low signal intensity volume
- %MR-TSV:
-
Percentage total abnormal signal intensity volume
- 3D-UTE:
-
Three-dimensional high-resolution morphology using ultrashort echo-times
- CF:
-
Cystic fibrosis
- PFT:
-
Pulmonary function test
- SD:
-
Standard deviation
References
Bell SC, Mall MA, Gutierrez H et al (2020) The future of cystic fibrosis care: a global perspective. Lancet Respir Med 8:65–124. https://doi.org/10.1016/S2213-2600(19)30337-6
Burgel P-R, Bellis G, Olesen HV et al (2015) Future trends in cystic fibrosis demography in 34 European countries. Eur Respir J 46:133–141. https://doi.org/10.1183/09031936.00196314
Ronan NJ, Einarsson GG, Twomey M et al (2018) CORK study in cystic fibrosis: sustained improvements in ultra-low-dose chest CT scores after CFTR modulation with ivacaftor. Chest 153:395–403. https://doi.org/10.1016/j.chest.2017.10.005
Bhalla M, Turcios N, Aponte V et al (1991) Cystic fibrosis: scoring system with thin-section CT. Radiology 179:783–788. https://doi.org/10.1148/radiology.179.3.2027992
Helbich TH, Heinz-Peer G, Eichler I et al (1999) Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology 213:537–544. https://doi.org/10.1148/radiology.213.2.r99nv04537
Brody AS, Klein JS, Molina PL, Quan J, Bean JA, Wilmott RW (2004) High-resolution computed tomography in young patients with cystic fibrosis: distribution of abnormalities and correlation with pulmonary function tests. J Pediatr 145:32–38. https://doi.org/10.1016/j.jpeds.2004.02.038
Calder AD, Bush A, Brody AS, Owens CM (2014) Scoring of chest CT in children with cystic fibrosis: state of the art. Pediatr Radiol 44:1496–1506. https://doi.org/10.1007/s00247-013-2867-y
Chassagnon G, Hubert D, Fajac I, Burgel PR, Revel MP (2016) Long-term computed tomographic changes in cystic fibrosis patients treated with ivacaftor. Eur Respir J 48:249–252. https://doi.org/10.1183/13993003.01918-2015
Rosenow T, Oudraad MCJ, Murray CP et al (2015) PRAGMA-CF. A quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med 191:1158–1165. https://doi.org/10.1164/rccm.201501-0061OC
Wielpütz MO, Weinheimer O, Eichinger M et al (2013) Pulmonary emphysema in cystic fibrosis detected by densitometry on chest multidetector computed tomography. PLoS One 8:e73142. https://doi.org/10.1371/journal.pone.0073142
Chassagnon G, Martin C, Burgel P-R et al (2018) An automated computed tomography score for the cystic fibrosis lung. Eur Radiol 28:5111–5120. https://doi.org/10.1007/s00330-018-5516-x
Leuraud K, Richardson DB, Cardis E et al (2015) Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol 2:e276–e281. https://doi.org/10.1016/S2352-3026(15)00094-0
Altes TA, Eichinger M, Puderbach M (2007) Magnetic resonance imaging of the lung in cystic fibrosis. Proc Am Thorac Soc 4:321–327. https://doi.org/10.1513/pats.200611-181HT
Eichinger M, Optazaite D-E, Kopp-Schneider A et al (2012) Morphologic and functional scoring of cystic fibrosis lung disease using MRI. Eur J Radiol 81:1321–1329. https://doi.org/10.1016/j.ejrad.2011.02.045
Boiselle PM, Biederer J, Gefter WB, Lee EY (2013) Expert opinion: why is MRI still an under-utilized modality for evaluating thoracic disorders? J Thorac Imaging 28:137. https://doi.org/10.1097/RTI.0b013e31828cafe7
Johnson KM, Fain SB, Schiebler ML, Nagle S (2013) Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med 70:1241–1250. https://doi.org/10.1002/mrm.24570
Dournes G, Grodzki D, Macey J et al (2015) Quiet submillimeter MR imaging of the lung is feasible with a PETRA sequence at 1.5 T. Radiology 276:258–265. https://doi.org/10.1148/radiol.15141655
Roach DJ, Crémillieux Y, Fleck RJ et al (2016) Ultrashort echo-time magnetic resonance imaging is a sensitive method for the evaluation of early cystic fibrosis lung disease. Ann Am Thorac Soc 13:1923–1931. https://doi.org/10.1513/AnnalsATS.201603-203OC
Dournes G, Menut F, Macey J et al (2016) Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur Radiol 26:3811–3820. https://doi.org/10.1007/s00330-016-4218-5
Dournes G, Yazbek J, Benhassen W et al (2018) 3D ultrashort echo time MRI of the lung using stack-of-spirals and spherical k -space coverages: evaluation in healthy volunteers and parenchymal diseases: lung MRI with 3D UTE spiral VIBE sequence. J Magn Reson Imaging. https://doi.org/10.1002/jmri.26212
Benlala I, Berger P, Girodet P-O et al (2019) Automated volumetric quantification of emphysema severity by using ultrashort echo time MRI: validation in participants with chronic obstructive pulmonary disease. Radiology 292:216–225. https://doi.org/10.1148/radiol.2019190052
Centre de Reference Mucoviscidose (2017) Protocole National de Diagnostic et de Soin (PNDS) Mucoviscidose. Haute Autorité de Santé, France. Available via https://www.has-sante.fr/portail/upload/docs/application/pdf/2017-09/pnds_2017_vf1.pdf. Accessed 20 Oct 2019
Fuchs HJ, Borowitz DS, Christiansen DH et al (1994) Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 331:637–642. https://doi.org/10.1056/NEJM199409083311003
Grodzki DM, Jakob PM, Heismann B (2012) Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med 67:510–518. https://doi.org/10.1002/mrm.23017
Lynch DA, Austin JHM, Hogg JC et al (2015) CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology 277:192–205. https://doi.org/10.1148/radiol.2015141579
Armato SG III, Giger ML, Blackburn JT, Doi K, MacMahon H (1999) Three-dimensional approach to lung nodule detection in helical CT. In: Hanson KM (ed) , San Diego, pp 553–559
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246:697–722. https://doi.org/10.1148/radiol.2462070712
Stahl M, Wielpütz MO, Graeber SY et al(2017) Comparison of lung clearance index and magnetic resonance imaging for assessment of lung disease in children with cystic fibrosis. Am J Respir Crit Care Med 195:349–359. https://doi.org/10.1164/rccm.201604-0893OC
Ciet P, Serra G, Bertolo S et al (2016) Assessment of CF lung disease using motion corrected PROPELLER MRI: a comparison with CT. Eur Radiol 26:780–787. https://doi.org/10.1007/s00330-015-3850-9
Duan G, Zhao X, Anderson SW, Zhang X (2019) Boosting magnetic resonance imaging signal-to-noise ratio using magnetic metamaterials. Commun Phys 2:35. https://doi.org/10.1038/s42005-019-0135-7
Woods JC, Wild JM, Wielpütz MO et al (2019) Current state of the art MRI for the longitudinal assessment of cystic fibrosis. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27030
Pennati F, Salito C, Borzani I et al (2019) Quantitative multivolume proton-magnetic resonance imaging in patients with cystic fibrosis lung disease: comparison with clinical indicators. Eur Respir J 53. https://doi.org/10.1183/13993003.02020-2017
Acknowledgments
The study was achieved within the context of Laboratory of Excellence TRAIL ANR-10-LABX-57, and the authors would like to thank Dr. Wadie Benhassen, PhD, for technical support.
Funding
Gael Dournes received academic funding from the French Society of Radiology (Grant Alain Rahmouni SFR-CERF 2019) and IdeX Bordeaux (ANR-10-IDEX-03-02). Ilyes Benlala received academic funding from the Foundation Le Nouveau Souffle (AAP2018). Julie Macey received academic funding from the University Hospital of Bordeaux.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of the study is Dr. Gael Dournes.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Pr Patrick Berger has significant statistical expertise.
Informed consent
Written consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• Cross sectional study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1005 kb)
Rights and permissions
About this article
Cite this article
Benlala, I., Point, S., Leung, C. et al. Volumetric quantification of lung MR signal intensities using ultrashort TE as an automated score in cystic fibrosis. Eur Radiol 30, 5479–5488 (2020). https://doi.org/10.1007/s00330-020-06910-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06910-w