Abstract
Objective
Previous studies provided evidence that gadolinium can be found in the aqueous chamber (AC) of the eye several hours post injection (p.i.) of gadolinium-based contrast agents (GBCAs). This study aimed to investigate whether gadolinium can be detected promptly after injection of a macrocyclic GBCA on contrast-enhanced T1-weighted MRI in the AC of children.
Methods
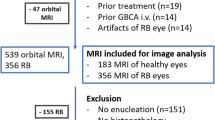
This retrospective study encompassed MRI of 200 healthy eyes of children suffering from retinoblastoma of the contralateral eye. MRI was performed with an orbital coil with the children in a state of general anesthesia. Differences of signal intensity ratios (∆SIRs) of the AC to the lens were determined between pre and post contrast-enhanced T1-weighted images (Dotarem®, Guerbet, 0.1 ml/kg body weight, mean (standard deviation) p.i. time = 12:24 (± 2:31) min).
Results
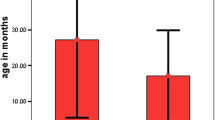
A highly significant signal intensity increase was found in the AC of healthy eyes 12 min after GBCA injection (median ∆SIR (interquartile range) = + 0.08 (0.05–0.12), p < 0.0001). In addition, gadolinium enhancement showed a strong negative correlation with children’s age in multivariate analysis with adjustment for p.i. time (p < 0.0001).
Conclusions
GBCA leakage into the AC of healthy infantile eyes was found promptly after injection. The negative correlation between patient age and GBCA enhancement might be explained by a maturation process of the blood-aqueous barrier or Schlemm’s canal. Future studies should assess the duration and potential diagnostic applications as well as possible safety concerns of gadolinium presence in the AC.
Key Points
• Leakage of gadolinium-based contrast agent into the aqueous chamber of infantile eyes was found promptly after intravenous injection (p < 0.0001).
• Gadolinium enhancement of the anterior eye chamber was negatively correlated with the children’s age (p < 0.0001).




Similar content being viewed by others
Abbreviations
- ∆SIR:
-
Difference of anterior chamber-to-lens signal intensity ratio
- AC:
-
Aqueous chamber / anterior chamber
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- FoV:
-
Field of view
- GBCA:
-
Gadolinium-based contrast agent
- GS:
-
Glymphatic system
- IQR:
-
Interquartile range
- MRI:
-
Magnetic resonance imaging
- p.i.:
-
Post injection
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- SI:
-
Signal intensity
- SIR:
-
Anter chamber-to-lens signal intensity ratio
References
Taoka T, Jost G, Frenzel T, Naganawa S, Pietsch H (2018) Impact of the glymphatic system on the kinetic and distribution of gadodiamide in the rat brain. Invest Radiol 53(9):529–534. https://doi.org/10.1097/RLI.0000000000000473
Jost G, Frenzel T, Lohrke J, Lenhard DC, Naganawa S, Pietsch H (2017) Penetration and distribution of gadolinium-based contrast agents into the cerebrospinal fluid in healthy rats: a potential pathway of entry into the brain tissue. Eur Radiol 27(7):2877–2885. https://doi.org/10.1007/s00330-016-4654-2
Lohrke J, Frisk A, Frenzel T et al (2017) Histology and gadolinium distribution in the rodent brain after the administration of cumulative high doses of linear and macrocyclic gadolinium-based contrast agents. Invest Radiol 52(6):324–333. https://doi.org/10.1097/RLI.0000000000000344
Deike-Hofmann K, Reuter J, Haase R et al (2019) Glymphatic pathway of gadolinium-based contrast agents through the brain: overlooked and misinterpreted. Invest Radiol 54(4):229–237. https://doi.org/10.1097/RLI.0000000000000533
Hitomi E, Simpkins AN, Luby M, Latour LL, Leigh RJ, Leigh R (2018) Blood–ocular barrier disruption in acute stroke patients. Neurology. 90(11):e915–e923. https://doi.org/10.1212/WNL.0000000000005123
Harrington D, D’Agostino RB, Gatsonis C et al (2019) New guidelines for statistical reporting in the journal. N Engl J Med 381(3):285–286. https://doi.org/10.1056/nejme1906559
Taoka T, Naganawa S (2018) Gadolinium-based contrast media, cerebrospinal fluid and the glymphatic system: possible mechanisms for the deposition of gadolinium in the brain. Magn Reson Med Sci 17(2):111–119. https://doi.org/10.2463/mrms.rev.2017-0116
Naganawa S, Nakane T, Kawai H, Taoka T (2017) Gd-based contrast enhancement of the perivascular spaces in the basal ganglia. Magn Reson Med Sci 16:61–65. https://doi.org/10.2463/mrms.mp.2016-0039
Iliff JJ, Lee H, Yu M et al (2013) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123(3):1299–1209. https://doi.org/10.1172/JCI67677
Kress BT, Iliff JJ, Xia M et al (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76(6):845–861. https://doi.org/10.1002/ana.24271
Ringstad G, Valnes LM, Dale AM et al (2018) Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insights 3(13):1–17. https://doi.org/10.1172/jci.insight.121537
Ringstad G, Vatnehol SAS, Eide PK (2017) Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 140(10):2691–2705. https://doi.org/10.1093/brain/awx191
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2013) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 270(3):834–841. https://doi.org/10.1148/radiol.13131669
Iliff JJ, Wang M, Liao Y et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid b. Sci Transl Med 4(147):ra111. https://doi.org/10.1126/scitranslmed.3003748
Bill A (1968) Capillary permeability to and extravascular dynamics of myoglobin, albumin and gammaglobulin in the uvea. Acta Physiol Scand 73(1–2):204–219. https://doi.org/10.1111/j.1748-1716.1968.tb04097.x
Raviola G (1977) The structural basis of the blood-ocular barriers. Exp Eye Res 25(1):27–63. https://doi.org/10.1016/S0014-4835(77)80009-2
Kinsey VE, Williamson MB (1949) Investigation of the blood-aqueous barrier in the newborn. II To inulin. Am J Ophthalmol 32(4):509–512. https://doi.org/10.1016/0002-9394(49)90875-2
Kinsey E, Blanche J, Terry TL (1945) Development of secretory function of ciliary body in the rabbit eye evaluated from ascorbic acid concentrations and changes in volume. J Gen Physiol 34(5):415–417. https://doi.org/10.1001/archopht.1945.00890190419013
Kinsey VE, Jackson B (1949) Investigation of the blood-aqueous barrier in the newborn. I. To ascorbic acid. Am J Ophthalmol 32(3):374–378. https://doi.org/10.1016/0002-9394(49)91930-3
Bembridge BA, Pirie A (2015) Biochemical and histological changes in developing rabbit eyes. Br J Ophthalmol 35(12):784–789. https://doi.org/10.1136/bjo.35.12.784
Kupfer C, Ross K (1971) The development of outflow facility in human eyes. Invest Ophthalmol 10(7):513–517 https://iovs.arvojournals.org/
Pandolfi M, Åstedt B (1971) Outflow resistance in the foetal eye. Acta Ophthalmol (Copenh) 49(2):344–350. https://doi.org/10.1111/j.1755-3768.1971.tb00959.x
Oshika T, Kato S, Funatsu H (1989) Quantitative assessment of aqueous flare intensity in diabetes. Graefes Arch Clin Exp Ophthalmol 227(6):518–520. https://doi.org/10.1007/BF02169443
Kubota T, Küchle M, Nguyen NX (1994) Aqueous flare in eyes with age-related macular degeneration. Jpn J Ophthalmol 38(1):67–70
Chen M-S, Hou P-K, Tai T-Y, Lin BJ (2008) Blood-ocular barriers. Tzu Chi Med J 20(1):25–34. https://doi.org/10.1016/S1016-3190(08)60004-X
Sawa M (2017) Laser flare-cell photometer: principle and significance in clinical and basic ophthalmology. Jpn J Ophthalmol 61(1):21–42. https://doi.org/10.1007/s10384-016-0488-3
Radbruch A, Roberts DR, Clement O, Rovira A, Quattrocchi CC (2017) Chelated or dechelated gadolinium deposition. Lancet Neurol 16(12):955. https://doi.org/10.1016/S1474-4422(17)30365-4
Radbruch A, Richter H, Fingerhut S et al (2019) Gadolinium deposition in the brain in a large animal model. Invest Radiol 54(9):531–536. https://doi.org/10.1097/RLI.0000000000000575
Robert P, Fingerhut S, Factor C et al (2018) One-year retention of gadolinium in the brain: comparison of gadodiamide and gadoterate meglumine in a rodent model. Radiology. 288(2):424–433. https://doi.org/10.1148/radiol.2018172746
Jost G, Frenzel T, Boyken J, Lohrke J, Nischwitz V, Pietsch H (2018) Long-term excretion of gadolinium-based contrast agents: linear versus macrocyclic agents in an experimental rat model. Radiology. 290(2):340–348. https://doi.org/10.1148/radiol.2018180135
Acknowledgments
We wish to acknowledge the help provided by PD Dr. Holland-Letz, Department of Biostatistics, German Cancer Research Center.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Alexander Radbruch.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
Alexander Radbruch: Guerbet, Bayer, GE.
Katerina Deike-Hofmann: Guerbet, Bayer, GE.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deike-Hofmann, K., von Lampe, P., Schlemmer, HP. et al. The anterior eye chamber: entry of the natural excretion pathway of gadolinium contrast agents?. Eur Radiol 30, 4633–4640 (2020). https://doi.org/10.1007/s00330-020-06762-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06762-4