Abstract
Objectives
The aim of the study is to compare coronal spectrally fat-suppressed 2D turbo spin-echo (TSE) with 2D short-tau inversion-recovery (STIR) sequences for the detection of optic nerve hyperintensities in patients with acute optic nerve neuritis (ON).
Methods
A retrospective review of patients with suspected unilateral ON and pathological visual evoked potentials, who received coronal TSE and STIR sequences with similar fast and clinically feasible acquisition times in addition to our standard imaging protocol. All images were evaluated and compared concerning the presence of optic nerve lesions, lesion lengths, and signal intensities in different anatomical parts of the optic nerves and CNR measures. A summary confidence score (CS) was calculated based on each reader’s subjective confidence regarding the scoring items.
Results
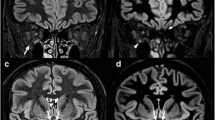
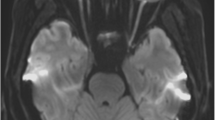
Interobserver agreements regarding the detection of optic nerve lesions were excellent for both sequences (TSE, κ = 0.89 and STIR, κ = 0.80). Greater extensions (17.4 ± 6.3 mm vs. 14.1 ± 5.8 mm), as well as higher numbers of optic nerve lesions in symptomatic nerves, were detected on TSE (49/52) compared with STIR (45/52) sequences (both p < 0.001). Overall CS were significantly (p < 0.001) higher for TSE (2.8) compared with STIR (2.1) sequences regarding the presence or absence of optic nerve lesions. CNR ratios of lesions’ mean signal intensities vs. ipsilateral surrounding orbital fat and vs. signal intensity measurements from contralateral optic nerves were significantly higher on TSE compared with STIR (p < 0.001 for both comparisons).
Conclusion
Spectrally fat-suppressed coronal 2D TSE sequences appear to be more sensitive for the detection of hyperintense optic lesions compared with 2D STIR sequences.
Key Points
• Spectrally fat-suppressed TSE sequences showed higher detection rates of hyperintense optic nerve lesions, as well as a higher reader confidence scores compared with STIR.
• Optic nerve signal abnormalities on TSE sequences were brighter and showed a greater expansion along the optic nerve course.
• CNR measures were significantly higher on TSE compared with STIR, when comparing the ratios of mean signal intensities of optic nerve lesions to ipsilateral orbital fat and to contralateral healthy optic nerves of both sequences.



Similar content being viewed by others
Abbreviations
- AQP4-IgG:
-
Aquaporin-4 immunoglobulin G
- AU:
-
Arbitrary unit
- CHESS:
-
Chemical shift selective
- CIS:
-
Clinically isolated syndrome
- CS:
-
Compound confidence score
- CSF:
-
Cerebrospinal fluid
- IQR:
-
Interquartile range
- L-CON-Q:
-
Lesion contralateral optic nerve quotient
- MRI:
-
Magnetic resonance imaging
- MS:
-
Multiple sclerosis
- NMOSD:
-
Neuromyelitis optica spectrum disorder
- ON:
-
Optic neuritis
- PACS:
-
Picture archiving and communication system
- ROI:
-
Region of interest
- SE:
-
Spin-echo
- SPIR:
-
Selective-partial inversion-recovery
- STIR:
-
Short-tau inversion-recovery
- TSE:
-
Turbo spin-echo
- VEP:
-
Visual evoked potentials
- WM:
-
White matter
References
Toosy AT, Mason DF, Miller DH (2014) Optic neuritis. Lancet Neurol 13:83–99
Wilhelm H, Schabet M (2015) The diagnosis and treatment of optic neuritis. Dtsch Arztebl Int 112:616–625 quiz 626
Dutra BG, da Rocha AJ, Nunes RH, Maia ACM Júnior (2018) Neuromyelitis optica spectrum disorders: spectrum of MR imaging findings and their differential diagnosis. Radiographics 38:169–193
Wingerchuk DM, Banwell B, Bennett JL et al (2015) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85:177–189
Hickman SJ, Miszkiel KA, Plant GT, Miller DH (2005) The optic nerve sheath on MRI in acute optic neuritis. Neuroradiology 47:51–55
Becker M, Masterson K, Delavelle J, Viallon M, Vargas MI, Becker CD (2010) Imaging of the optic nerve. Eur J Radiol 74:299–313
Jenkins TM, Toosy AT (2017) Optic neuritis: the eye as a window to the brain. Curr Opin Neurol 30:61–66
Guy J, Mao J, Bidgood WD Jr, Mancuso A, Quisling RG (1992) Enhancement and demyelination of the intraorbital optic nerve. Fat suppression magnetic resonance imaging. Ophthalmology 99:713–719
Hendrix LE, Kneeland JB, Haughton VM et al (1990) MR imaging of optic nerve lesions: value of gadopentetate dimeglumine and fat-suppression technique. AJR Am J Roentgenol 155:849–854
Johnson G, Miller DH, MacManus D et al (1987) STIR sequences in NMR imaging of the optic nerve. Neuroradiology 29:238–245
Onodera M, Yama N, Hashimoto M et al (2016) The signal intensity ratio of the optic nerve to ipsilateral frontal white matter is of value in the diagnosis of acute optic neuritis. Eur Radiol 26:2640–2645
Hodel J, Outteryck O, Bocher AL et al (2014) Comparison of 3D double inversion recovery and 2D STIR FLAIR MR sequences for the imaging of optic neuritis: pilot study. Eur Radiol 24:3069–3075
Riederer I, Mühlau M, Hoshi MM, Zimmer C, Kleine JF (2019) Detecting optic nerve lesions in clinically isolated syndrome and multiple sclerosis: double-inversion recovery magnetic resonance imaging in comparison with visually evoked potentials. J Neurol 266:148–156
Jackson A, Sheppard S, Laitt RD, Kassner A, Moriarty D (1998) Optic neuritis: MR imaging with combined fat- and water-suppression techniques. Radiology 206:57–63
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Siewert C, Hosten N, Felix R (1994) The use of the T2-weighted turbo-spin-echo sequence in studying the neurocranium. A comparison with the conventional T2-weighted spin-echo sequence. Rofo 161:44–50
de Kerviler E, Leroy-Willig A, Clément O, Frija J (1998) Fat suppression techniques in MRI: an update. Biomed Pharmacother 52:69–75
Keller PJ, Hunter WW Jr, Schmalbrock P (1987) Multisection fat-water imaging with chemical shift selective presaturation. Radiology 164:539–541
Naganawa S, Koshikawa T, Nakamura T et al (2004) Comparison of flow artifacts between 2D-FLAIR and 3D-FLAIR sequences at 3 T. Eur Radiol 14:1901–1908
Aiken AH, Mukherjee P, Green AJ, Glastonbury CM (2011) MR imaging of optic neuropathy with extended echo-train acquisition fluid-attenuated inversion recovery. AJNR Am J Neuroradiol 32:301–305
Onofrj M, Tartaro A, Thomas A et al (1996) Long echo time STIR sequence MRI of optic nerves in optic neuritis. Neuroradiology 38:66–69
Tartaro A, Onofrj M, Delli Pizzi C et al (1996) Long time echo STIR sequence magnetic resonance imaging of optic nerves in optic neuritis. Ital J Neurol Sci 17:35–42
Voss E, Raab P, Trebst C, Stangel M (2011) Clinical approach to optic neuritis: pitfalls, red flags and differential diagnosis. Ther Adv Neurol Disord 4:123–134
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Dr. med. Jens Fiehler, Hamburg.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Dr. Stellmann reports grants from Alpina, during the conduct of the study; grants and personal fees from Biogen; personal fees from Genzyme; and grants from Novartis, outside the submitted work. These fundings did not compromise or influence the scientific work presented in this manuscript.
Statistics and biometry
Dr. Uta Hanning and Dr. Fabian Flottmann have significant statistical expertise. No complex statistical methods were necessary for this paper.
Informed consent
The study was approved by the local research Ethical Committee Hamburg (Ethik-Komission der Ärztekammer Hamburg), following the guidelines of the Declaration of Helsinki and written informed consent was given from every participant.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Faizy, T.D., Broocks, G., Frischmuth, I. et al. Spectrally fat-suppressed coronal 2D TSE sequences may be more sensitive than 2D STIR for the detection of hyperintense optic nerve lesions. Eur Radiol 29, 6266–6274 (2019). https://doi.org/10.1007/s00330-019-06255-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06255-z