Abstract
Objectives
To prospectively investigate concordance between whole-body MRI (WB-MRI) and a composite reference standard for initial staging and interim response evaluation in paediatric and adolescent Hodgkin’s lymphoma.
Methods
Fifty patients (32 male, age range 6–19 years) underwent WB-MRI and standard investigations, including 18F-FDG-PET-CT at diagnosis and following 2–3 chemotherapy cycles. Two radiologists in consensus interpreted WB-MRI using prespecified definitions of disease positivity. A third radiologist reviewed a subset of staging WB-MRIs (n = 38) separately to test for interobserver agreement. A multidisciplinary team derived a primary reference standard using all available imaging/clinical investigations. Subsequently, a second multidisciplinary panel rereviewed all imaging with long-term follow-up data to derive an enhanced reference standard. Interobserver agreement for WB-MRI reads was tested using kappa statistics. Concordance for correct classification of all disease sites, true positive rate (TPR), false positive rate (FPR) and kappa for staging/response agreement were calculated for WB-MRI.
Results
There was discordance for full stage in 74% (95% CI 61.9–83.9%) and 44% (32.0–56.6%) of patients against the primary and enhanced reference standards, respectively. Against the enhanced reference standard, the WB-MRI TPR, FPR and kappa were 91%, 1% and 0.93 (0.90–0.96) for nodal disease and 79%, < 1% and 0.86 (0.77–0.95) for extra-nodal disease. WB-MRI response classification was correct in 25/38 evaluable patients (66%), underestimating response in 26% (kappa 0.30, 95% CI 0.04–0.57). There was a good agreement for nodal (kappa 0.78, 95% CI 0.73–0.84) and extra-nodal staging (kappa 0.60, 95% CI 0.41–0.78) between WB-MRI reads
Conclusions
WB-MRI has reasonable accuracy for nodal and extra-nodal staging but is discordant with standard imaging in a substantial minority of patients, and tends to underestimate disease response.
Key Points
• This prospective single-centre study showed discordance for full patient staging of 44% between WB-MRI and a multi-modality reference standard in paediatric and adolescent Hodgkin’s lymphoma.
• WB-MRI underestimates interim disease response in paediatric and adolescent Hodgkin’s lymphoma.
• WB-MRI shows promise in paediatric and adolescent Hodgkin’s lymphoma but currently cannot replace conventional staging pathways including 18F-FDG-PET-CT.
Similar content being viewed by others
Introduction
Hodgkin’s lymphoma (HL) is the most common adolescent lymphoma [1]. Positron emission tomography computed tomography (18F-FDG-PET-CT) remains the first-line imaging technique [2], providing both structural and functional metabolic information to localise and characterise tumour burden. Furthermore, as a biomarker of glucose metabolism, uptake of the radiotracer 18F-2-fluro-2-deoxy-d-glucose (18F-FDG) provides a more accurate assessment of treatment response than simple structural evaluation [2,3,4]. 18F-FDG-PET-CT, however, imparts a substantial dose of ionising radiation, which may be associated with increased risk of secondary malignancies [2, 5]. This is a concern in the paediatric age group given the increased sensitivity of tissues to radiation exposure, coupled with the significant improvement in long-term survival [6, 7].
Whole-body magnetic resonance imaging (WB-MRI) is an attractive alternative to 18F-FDG-PET-CT as it does not impart ionising radiation and can provide high quality anatomical images through the body in less than 1 h [6, 8, 9]. Moreover, there is evidence suggesting that diffusion-weighted imaging (DWI) may act as a surrogate for the functional information provided by 18F-FDG [10, 11]. DWI captures water movement within tissue and its functional parameter, the apparent diffusion coefficient (ADC), is a marker of tissue cellularity and related to glucose metabolism [12, 13].
There is increasing supportive literature for implementation of WB-MRI in lymphoma staging pathways [14,15,16,17,18], although such data remains relatively sparse in the paediatric population [19]. Extrapolation from adult studies may be flawed given the complexities of imaging patients with smaller body habitus, the challenges of prolonged WB-MRI protocols for younger patients, and potential differences in disease patterns and behaviours between paediatric and adult patients, and within lymphoma subtypes.
The purpose of this study was to investigate prospectively the concordance between WB-MRI and a composite reference standard based on clinical evaluation, histology and standard staging imaging including 18F-FDG-PET-CT for initial staging and interim treatment response monitoring in paediatric and adolescent Hodgkin’s lymphoma.
Material and methods
We conducted a prospective single-arm cohort study in a single tertiary referral centre, following ethical permission (Clinicaltrials.gov number: NCT01459224).
Consent for study investigations, including collection of anonymised patient data, was obtained from patients and or their parents/guardians according to the institutional and ethical committee guidelines.
Patient population
Consecutive patients were prospectively identified between December 2011 and August 2014 inclusive from the paediatric lymphoma service of University College London Hospital.
Inclusion criteria were age 5–20 years (inclusive), histological confirmation of HL or clinically suspected HL (classical HL and nodular lymphocyte predominant HL) undergoing staging investigations pending final biopsy confirmation, and patients/guardian consent. All patients were either recruited to the Euronet PHL-C1 or PHL-LP1 trials [20] or were due to undergo treatment using the chemotherapy regimens of these trials.
Exclusion criteria included previous diagnosis of HL without being disease free for 5 years, previous chemotherapy and or radiotherapy within the previous 2 years, pregnancy or breastfeeding and any known contraindication to MRI.
Summary of study conduct
All recruited patients underwent the standard staging investigations employed at the recruiting institution: (i) whole-body 18F-FDG-PET-CT, (ii) anatomical WB-MRI sequences with single-phase post-contrast acquisition through the upper abdomen, (iii) abdominal ultrasound in cases of equivocal solid organ involvement and (iv) contrast-enhanced chest CT (CE chest CT) scan in case of equivocal lung involvement.
To provide a comprehensive “stand-alone” WB-MRI protocol, for the purposes of the current study, the WB-MRI protocol was extended to include whole-body DWI and dynamic contrast-enhanced (DCE) sequences though the liver/spleen and chest, as well as the standard basic anatomical sequences.
Thereafter, patients underwent interim 18F-FDG-PET-CT (iPET-CT) within 14 days of completing the first two (Euronet PHL-C1) or three (Euronet LP1) cycles of chemotherapy for initial treatment response evaluation. Patients were invited to undergo a second WB-MRI (iWB-MRI) and were followed for a minimum 24 months post chemotherapy.
Staging imaging interpretation
A full description of WB-MRI and standard imaging interpretation is provided in the ESM. In brief, 18F-FDG-PET-CT was interpreted by a nuclear medicine physician (LM with more than 10 years of experience) and basic anatomical WB-MRI sequences including single-phase post-contrast sequences through the upper abdomen (but excluding DWI and DCE sequences), abdominal ultrasound and chest CT images (when available) were evaluated by consultant paediatric radiologist (PH with 11 years of experience in WB-MR imaging). WB-MRI was interpreted by two radiologists (SAT and SP with 5 and 7 years’ experience of WB-MRI) in consensus utilising all the available sequences (including the DWI and DCE images). The radiologists were blinded to the clinical history (other than the diagnosis of lymphoma) and all other investigations. A third blinded radiologist (MK with 3 years’ experience of WB-MRI) interpreted a subset of 38 WB-MRI data sets to test interobserver agreement with the primary consensus read.
The disease status for 18 nodal and 14 extra-nodal sites was evaluated, as well as the final Ann Arbor stage derived using predefined definitions based on size, 18F-FDG uptake and ADC (based on previous pilot data [21]; see ESM).
Interim treatment response evaluation
Interim 18F-FDG-PET-CT (iPET-CT) and WB-MRI (iWB-MRI) were interpreted by the same individuals who read the initial staging investigations.
For WB-MRI, the ADC criteria for nodal response were based on those derived from previous work investigating ADC changes in responsive and non-responsive nodal disease [21] (Table 1). Extra-nodal response was evaluated by qualitative assessment of iWB-MRI classifying response into four categories: (a) locally undetectable (complete response), (b) locally detectable but reduction in size or number of deposits (partial response), (c) locally unchanged (no change in the number or size of deposits) and (d) locally progressive (increase in size or number of deposits).
A full description of interim response evaluation is provided in ESM.
Primary and enhanced reference standards
A full description is provided in ESM. In brief, the primary reference standard was assigned by a multidisciplinary team (MDT) on the basis of their assessment of all standard imaging tests together with all clinical information, including available histology.
Given the potential limitations of standard imaging in staging HL, which may weaken the primary reference standard, a retrospective enhanced reference standard was also produced by a central expert panel comprising two radiologists, two nuclear medicine physicians and two paediatric haemato-oncologists. The central panel reviewed all staging, interim and end of treatment scans as well as follow-up imaging and clinical outcomes up to 24 months post chemotherapy. The panel corrected simple labelling (boundary) discrepancies that were due to differences in disease site description between tests, and thereafter any perceptual or technical failures in the primary reference standard (Fig. 1). WB-MRI perceptual errors were also noted.
Example of 18F-FDG-PET-CT perceptual error. Right axillary nodal station was called negative on (a) 18F-FDG-PET-CT and positive on WB-MRI. b500 diffusion-weighted MRI (b) and apparent diffusion coefficient map (c) showing restricted diffusion (ADC 1.0 × 10−3 mm2/s). On retrospective evaluation of nodal station, with full follow-up data available, the expert panel judged the right axillary node (arrows) to be a positive nodal site based on 18F-FDG uptake, and thus a perceptual error on initial 18F-FDG-PET-CT interpretation
Data analysis and study power
The primary endpoint was based on achieving full (100%) concordance between WB-MRI and the primary reference standard in terms of correct disease classification for each and every anatomical site (i.e. the 18 nodal and 14 extra-nodal sites). A binary classification of each disease status as either negative or positive/equivocal was made as part of the reference standard.
See ESM for the power calculation of the study sample size.
The primary endpoint was summarised in terms of frequency and percentage of patients who had a concordance below 100% for all disease sites combined, and separately for nodal and extra-nodal sites. The median and interquartile range (IQR) discordance rate for each patient was also calculated.
The true positive rate (TPR) (sensitivity) and false positive rate (FPR) of WB-MRI were calculated for nodal and extra-nodal disease sites, along with the kappa statistic. Agreement for Ann Arbor staging and classification of interim treatment response evaluation (for positive/equivocal disease sites that were concordant at initial staging) were summarised in terms of frequency, percentages and kappa.
Sensitivity analysis were performed using the outcomes from the central review process, and the enhanced reference standard.
Specifically, the agreement analyses for staging WB-MRI were repeated:
-
After correcting for anatomical boundary labelling description discrepancies only
-
Against the enhanced reference standard (including correction of boundary labelling description discrepancies)
-
Against the enhanced reference standard after removal of WB-MRI perceptual errors
Ann Arbor staging agreement was also assessed after integrating the results of enhanced reference standard and after WB-MRI correction for perceptual errors.
Interobserver agreement between consensus WB-MRI read and the third radiologist was tested using kappa statistics.
Statistical analysis was performed using the Stata software package (Version 14. Stata Corporation LP, College Station, Texas).
Results
Patient characteristics
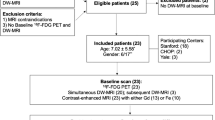
Fifty-eight patients were recruited (M/F 39:19, median age 16, range 5–19 years). The study flowchart is presented in Fig. 2. Eight patients were excluded. The demographics, disease subtype and treatment regimen of the final 50 patient study cohort are shown in Table 2. Staging WB-MRI was performed within a median 2 days (range 0–20 days) of 18F-FDG-PET-CT without any complication, and before treatment in all patients.
Central review and enhanced reference standard
Across the cohort there were 1527 disease sites [875 nodal (850 predefined sites and 25 “other” sites and 652 extra-nodal sites (650 predefined sites and 2 “other” sites)] evaluated by both WB-MRI and standard imaging.
The central review identified and resolved 44 anatomical boundary labelling description discrepancies. There were 10 nodal and 4 extra-nodal perceptual errors in the primary reference standard, together with 1 technical error.
There were 20 WB-MRI perceptual errors.
Initial staging agreement: per patient
Per patient concordance rate for each analysis is shown in Table 3 and Fig. 3.
Per patient concordance rate. Concordance rate for nodal, extra-nodal and combined nodal/extra-nodal sites between a WB-MRI and primary reference standard prior to the removal of simple boundary classification labelling discrepancies and b following the removal of simple boundary classification labelling discrepancies. c WB-MRI and the enhanced reference standard (following removal of 18F-FDG-PET-CT perceptual and technical errors) and d WB-MRI and the enhanced reference standard following removal of WB-MRI perceptual errors. Median and interquartile range (IQR) are presented for each analysis tier
After correcting for labelling discrepancies, the discordance rate was 44% (90% CI exact 32.0–56.6%) for nodal sites and 28% (90% CI exact 17.8–40.3%) for extra-nodal sites.
Against the enhanced reference standard, the equivalent discordance rates fell to 44% (90% CI exact 32.0–56.6%) for all sites, 34% (90% CI exact 23.0–46.5%) for nodal sites and 18% (90% CI exact 9.7–29.3%) for extra-nodal sites. After removal of WB-MRI perceptual errors, the discordance rates for all, nodal and extra-nodal sites were 18% (90% CI exact 9.7–29.3%), 16% (90% CI exact 8.2–27.0%) and 4% (90% CI exact 0.7–12.1%), respectively.
Initial staging agreement: disease site
Absolute agreement rate, TPR, FPR and Cohen’s kappa statistic for nodal and extra-nodal disease sites for each analysis are shown in Table 4.
Against the enhanced reference standard, the WB-MRI TPR, FPR and kappa agreement were 91%, 1% and 0.93 (95% CI 0.90–0.96) for nodal disease and 79%, < 1% and 0.86 (95% CI 0.77–0.95) for extra-nodal disease.
Following removal of WB-MRI perceptual errors, the TPR, FPR and kappa agreement were 97%, < 1% and 0.97 (95% CI 0.95–0.99) for nodal and 95%, 0% and 0.97 (95% CI 0.93–1.00) for extra-nodal assessment compared to enhanced reference standard. There were seven WB-MRI false negative nodal sites due to technical failures (i.e. not visible in retrospect), two false positive nodal sites and two false negative extra-nodal sites (Supplemental Table 4).
Ann Arbor staging agreement
Based on enhanced reference standard, there were 2, 26, 5, 14 and 3 patients with Ann Arbor stage 1, 2, 3, 4 and 4E, respectively.
Agreement between WB-MRI and the primary reference standard was substantial (kappa 0.66, 95% CI 0.50–0.83) with staging concordant in 39/50 (78%) patients (Supplemental Table 5).
Prior to removal of WB-MRI perceptual errors, agreement between WB-MRI and the enhanced reference was substantial (kappa 0.72, 95% CI 0.56–0.88) with concordance in 41/50 (82%) patients. After removal of the WB-MRI perceptual errors concordance was achieved in 48/50 patients (96%), (kappa 0.94, 95% CI 0.85–1.00). Two patients were under-staged as a result of technical failure of WB-MRI compared to enhanced reference (Fig. 4 and Supplemental Table 5).
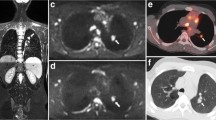
Example of WB-MRI technical error. False negative WB-MRI technical error resulting in under-staging of a 15-year-old female patient with multifocal bone marrow involvement; a axial STIR-HASTE, b DWI b500 and c coronal STIR-HASTE MRI show no discernible bone marrow abnormality. d 18F-FDG-PET-CT, however, demonstrates multifocal bone marrow metastasis (arrows)
Interim treatment response agreement
Thirty-eight of the 50 patients were evaluable for interim treatment response analysis (Fig. 2). iWB-MRI scans were acquired within a median 1 day (range 0–7 days) of iPET scans.
On a per patient basis, iWB-MRI agreed with the primary reference standard response classification in 25/38 patients (66%, 6 PR and 19 CR), underestimating response in 10 (26%) patients and overestimating response in 3 (8%) patients (kappa 0.30, 95% CI 0.04–0.57) (Table 5).
There were 143 nodal and 26 extra-nodal positive concordant sites evaluable for interim treatment assessment.
iWB-MRI agreed with the primary reference standard response classification in 126/143 (88%) nodal sites, underestimating response in 3 (2%) sites and overestimating response in 14 (10%) (Supplemental Table 6).
iWB-MRI agreed with primary reference standard response classification in 17/26 (66%) of extra-nodal sites. In the remaining 9 (34%) sites, WB-MRI underestimated response (Fig. 5). Specifically, WB-MRI underestimated bone marrow response in four patients (three with reduced but persistent detectable disease and one with unchanged disease), and for spleen and lung in two and three patients respectively (all five with reduced but persistent disease on WB-MRI). All nine sites showed complete response on primary reference standard.
Example of discrepant interim treatment response classification. WB-MRI and 18F-FDG-PET-CT of an 8-year-old male subject with Ann Arbor stage 4 disease. Baseline WB-MRI (a) and 18F-FDG-PET-CT (c) showing involvement of entire T11 vertebrae (arrows). Interim WB-MRI (b) showing no signal intensity changes (arrow) whilst interim 18F-FDG-PET-CT (d) demonstrated complete response. Patient remained in remission following chemotherapy
WB-MRI interobserver agreement
There was a good agreement between the consensus WB-MRI and the 3rd radiologist reads for nodal (kappa 0.78, 95% CI 0.73–0.84), extra-nodal staging (kappa 0.60, 95% CI 0.41–0.78) and Ann Arbor staging (kappa 0.62, 95% CI 0.32–0.73).
Discussion
In the current study we compared WB-MRI with a combined multi-modality reference standard based mainly on standard imaging (notably 18F-FDG-PET-CT) but including clinical and histological data for staging and interim treatment response monitoring in paediatric HL.
Overall, we found that WB-MRI has reasonable accuracy for nodal and extra-nodal staging but did not achieve full concordance for all disease sites in a substantial minority of patients, and tends to underestimate disease response.
Our findings of intrinsically high sensitivity and specificity for nodal and extra-nodal staging confirm the data of Littooij et al. who performed a similar staging study in a cohort of 33 paediatric patients with a range of lymphoma phenotypes [19], and mirror those of Mayerhoefer et al. [17] who studied a cohort of 140 adult patients. In line with previous work [14], we utilised a rigorous consensus review process taking into consideration all long-term imaging and clinical follow-up to create an enhanced reference standard, thereby correcting deficiencies in standard staging pathways, and providing a more realistic evaluation of the accuracy of WB-MRI. Against this enhanced reference, WB-MRI, sensitivity for extra-nodal disease was still modest at 79%. We also retrospectively corrected WB-MRI perceptual errors to indicate the theoretical “best” technical performance of WB-MRI, which increased nodal sensitivity to 97% and extra-nodal disease sensitivity to 95%. Clearly perceptual errors are unavoidable so such corrected data will overestimate the performance of WB-MRI, but particular emphasis should be made on detecting extra-nodal disease during radiologist training.
Our primary analysis, and one rarely performed in the literature, is how often WB-MRI achieved full concordance with standard imaging for each and every disease site in an individual patient. Such data is clinically highly relevant, as patients with early unfavourable response will often undergo targeted radiotherapy to individual involved nodal stations following chemotherapy [22]. Against the enhanced reference standard, full concordance for nodal disease was achieved in 66% of patients, which increased to 84% after removal of WB-MRI perceptual errors. Although such data is encouraging, there is a substantial minority of patients with discordant findings to standard staging, which may have treatment implications. Our data suggests that using ADC as a surrogate for 18F-FDG uptake, although promising [12, 21], is currently insufficient. It is clear there is overlap in ADC between malignant lymph nodes and normal/reactive lymph nodes and the optimal ADC cut-off remains unclear, and requires further investigation [23].
Although access to new 18F-FDG-PET-MR technology is currently very limited, this platform my ultimately prove to be the investigation of choice and prospective studies are currently underway [24].
The accuracy of iWB-MRI for interim treatment response assessment is under investigation, but far from proven [11,12,13, 18, 25].
Using simple visual inspection of DWI images, Mayerhoefer et al. [18] reported that region-based agreement between WB-DWI with 18F-FDG-PET-CT was 99.2% after 1–3 therapy cycles in their cohort of 51 adult patients with various lymphoma types, and Tsuji et al. [11] found that WB-DWI was concordant with 18F-FDG-PET-CT in 100% of cases (n = 19) with lesion negative interim scans.
One potential advantage of applying quantitative ADC cut-offs for response assessment is to improve the specificity of simple visual assessment. Littooij et al. [13], for example, reported that applying an ADC cut-off value of 1.21 × 10−3 mm2/s increased specificity for residual nodal disease detection by nearly 30% compared to visual inspection only.
By applying a similar ADC cut-off, we found that iWB-MRI agreed with the reference standard in a moderate 66% of patients.
One particular observation was the persistence of abnormal DWI bone marrow signal after successful treatment, resulting in underestimation of response by MRI and highlighting a limitation of visual response of extra-nodal disease on DWI. Quantitative ADC measurements my aid the differentiation between persistent tumour and treatment necrosis [26] and requires further investigation. For example, post-chemotherapy ADC monitoring in multiple myeloma has already shown promise for response assessment [27]. Such evidence is currently lacking in paediatric lymphoma, although intuitively ADC assessment could also be beneficial, and requires further evaluation.
Our study has some limitations. Our standard staging protocol, although primarily based on 18F-FDG-PET-CT, also includes anatomical MRI sequences. There is a theoretical risk of incorporation bias as these sequences were available to the MDT when they created the primary reference standard [28]. However, DWI and DCE sequences were not available to the MDT, and the complete WB-MRI examination was viewed as a standalone examination by radiologists blinded to all other clinical information. As noted, 18F-FDG-PET-CT is the mainstay of staging at our institution. Any incorporation bias would favour WB-MRI and the fact we report modest WB-MRI performance data suggests that any bias did not influence the overall study outcome.
We used an unblinded expert panel opinion and long-term follow-up data to derive the enhanced reference standard, an approach commonly used in studies of imaging diagnostic accuracy in absence of a single reference standard [14, 15].
We have used the highest b value of 500 s/mm2 for DWI disease assessment. We acknowledge that a higher b value between 800 and 1000 s/mm2 would have been in line with current recommendations on WB-DWI [29]. However, our ADC cut-off parameters were derived from previous pilot work [21] using similar DWI protocol as the current study. It is, however, possible that using a higher b value of 800–1000 s/mm2 instead of 500 s/mm2 could improve disease detection because of a superior lesion-to-contrast ratio. This could, for example, potentially decrease perceptual errors for extra-nodal disease assessment.
We used both qualitative and quantitative MRI assessment for staging and response monitoring. The generalizability of ADC quantitation across institutions and platforms, however, remains challenging [30, 31]. We also used a consensus reading paradigm for WB-MRI as at the time of the study set-up this mirrored our usual clinical practice and the use of ADC cut-offs was deemed exploratory [32]. We did reassuringly demonstrate good interobserver agreement with a third radiologist (as have others [19]). However, given that consensus reading is not widely used, it cannot be assumed that our data is representative of standard clinical practice where single reading is more common.
It has been shown that quantitative ADC changes following chemotherapy may differ between HL and non-HL subtypes of lymphoma [25] and our data is applicable to paediatric and adolescent HL.
Finally, although ADC changes as early as 1 week post chemotherapy have been documented for very early response assessment in adult lymphoma [33], the delayed second time point for iWB-MRI in our study was based on institutional guidelines for iPET-CT, Euronet trial [20] and recommendations in the literature [34, 35]. It would now be useful to investigate whether WB-MRI performs better for response assessment if performed at an earlier time point (e.g. 2 weeks) after chemotherapy.
In conclusion, WB-MRI with DWI has reasonable intrinsic diagnostic performance for nodal and extra-nodal staging of paediatric HL. However, in a substantial minority of patients it fails to achieve full concordance with standard imaging for all disease sites. WB-MRI has reasonable accuracy for interim treatment response classification but tends to underestimate disease response, particularly in extra-nodal disease sites. Overall, although promising, WB-MRI with DWI cannot currently replace standard imaging investigations in paediatric and adolescent Hodgkin’s lymphoma and further research is required, particularly to derive optimum ADC cut-offs for disease status, and the significance of persistent extra-nodal abnormality following treatment.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DWI:
-
Diffusion-weighted imaging
- FPR:
-
False positive rate
- HL:
-
Hodgkin’s lymphoma
- IQR:
-
Interquartile range
- MDT:
-
Multidisciplinary team
- TPR:
-
True positive rate
- WB-MRI:
-
Whole-body MRI
References
Ward E, DeSantis C, Robbins A, Kohler B, Jemal A (2014) Childhood and adolescent cancer statistics. CA Cancer J Clin 64:83–103
Uslu L, Doing J, Link M, Rosenberg J, Quon A, Daldrup-Link HE (2015) Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. J Nucl Med 56:274–286
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068
Hall EJ, Brenner DJ (2008) Cancer risks from diagnostic radiology. Br J Radiol 81:362–378
Nievelstein RA, Littooij AS (2016) Whole-body MRI in paediatric oncology. Radiol Med 121:442–453
Brenner D, Elliston C, Hall E, Berdon W (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 176:289–296
Davis JT, Kwatra N, Schooler GR (2016) Pediatric whole-body MRI: a review of current imaging techniques and clinical applications. J Magn Reson Imaging 44:783–793
Greer MC, Voss SD, States LJ (2017) Pediatric cancer predisposition imaging: focus on whole-body MRI. Clin Cancer Res 23:e6–e13
Maggialetti N, Ferrari C, Minoia C et al (2016) Role of WB-MR/DWIBS compared to (18)F-FDG PET/CT in the therapy response assessment of lymphoma. Radiol Med 121:132–143
Tsuji K, Kishi S, Tsuchida T et al (2015) Evaluation of staging and early response to chemotherapy with whole-body diffusion-weighted MRI in malignant lymphoma patients: A comparison with FDG-PET/CT. J Magn Reson Imaging 41:1601–1607
Punwani S, Taylor SA, Saad ZZ et al (2013) Diffusion-weighted MRI of lymphoma: prognostic utility and implication for PET/MRI? Eur J Nucl Med Mol Imaging 40:373–385
Littooij AS, Kwee TC, de Keizer B et al (2015) Whole-body MRI-DWI for assessment of residual disease after completion of therapy in lymphoma: a prospective multicenter study. J Magn Reson Imaging 42:1646–1655
Punwani S, Taylor SA, Bainbridge A et al (2010) Pediatric and adolescent lymphoma: comparison of whole-body STIR half-Fourier RARE MR imaging with an enhanced PET/CT reference for initial staging. Radiology 255:182–190
Kwee TC, Vermoolen MA, Akkerman EA et al (2014) Whole-body MRI, including diffusion-weighted imaging, for staging lymphoma: comparison with CT in a prospective multicenter study. J Magn Reson Imaging 40:26–36
Regacini R, Puchnick A, Shigueoka DC, Iared W, Lederman HM (2015) Whole-body diffusion-weighted magnetic resonance imaging versus FDG-PET/CT for initial lymphoma staging: systematic review on diagnostic test accuracy studies. Sao Paulo Med J 133:141–150
Mayerhoefer ME, Karanikas G, Kletter K et al (2014) Evaluation of diffusion-weighted MRI for pretherapeutic assessment and staging of lymphoma: results of a prospective study in 140 patients. Clin Cancer Res 20:2984–2993
Mayerhoefer ME, Karanikas G, Kletter K et al (2015) Evaluation of diffusion-weighted magnetic resonance imaging for follow-up and treatment response assessment of lymphoma: results of an 18F-FDG-PET/CT-controlled prospective study in 64 patients. Clin Cancer Res 21:2506–2513
Littooij AS, Kwee TC, Barber I et al (2014) Whole-body MRI for initial staging of paediatric lymphoma: prospective comparison to an FDG-PET/CT-based reference standard. Eur Radiol 24:1153–1165
Körholz D, Wallace H, Landman-Parker J (2006) EuroNet-Paediatric Hodgkin’s Lymphoma Group, first international inter-group study for classical Hodgkin’s lymphoma in children and adolescents, radiotherapy manual. https://www.skion.nl/workspace/uploads/euronet-phl-c1_workingcopy_inkl_amendm06_mw_2012-11-14_0.pdf. Accessed 02 Feb 2018
Punwani S, Prakash V, Bainbridge A et al (2010) Quantitative diffusion weighted MRI: a functional biomarker of nodal disease in Hodgkin’s lymphoma. Cancer Biomarker 7:249–259
Eich HT, Diehl V, Görgen H et al (2010) Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol 28:4199–4206
Vandecaveye V, De Keyzer F, Vander Poorten V et al (2009) Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology 251:134–146
Afaq A, Fraioli F, Sidhu H et al (2017) Comparison of PET/MRI with PET/CT in the evaluation of disease status in lymphoma. Clin Nucl Med 42:e1–e7
Hagtvedt T, Seierstad T, Lund KV et al (2015) Diffusion-weighted MRI compared to FDG PET/CT for assessment of early treatment response in lymphoma. Acta Radiol 56:152–158
Padhani AR, Koh DM, Collins DJ (2011) Whole-body diffusion-weighted MR imaging in cancer: current status and research directions. Radiology 261:700–718
Latifoltojar A, Hall-Craggs M, Bainbridge A et al (2017) Whole-body MRI quantitative biomarkers are associated significantly with treatment response in patients with newly diagnosed symptomatic multiple myeloma following bortezomib induction. Eur Radiol 27:5325–5336
Kohn MA, Carpenter CR, Newman TB (2013) Understanding the direction of bias in studies of diagnostic test accuracy. Acad Emerg Med 20:1194–1206
Barnes A, Alonzi R, Blackledge M et al (2018) UK quantitative WB-DWI technical workgroup: consensus meeting recommendations on optimisation, quality control, processing and analysis of quantitative whole-body diffusion-weighted imaging for cancer. Br J Radiol. https://doi.org/10.1259/bjr.20170577
Celik A (2016) Effect of imaging parameters on the accuracy of apparent diffusion coefficient and optimization strategy. Diagn Interv Radiol 22:101–107
Koh DM, Collins DJ, Orton MR (2011) Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol 196:1351–1361
Bankier AA, Levine D, Halpern EF, Kressel HY (2010) Consensus interpretation in imaging research: is there a better way? Radiology 257:14–17
Horger M, Claussen C, Kramer U, Fenchel M, Linchy M, Kaufmann S (2014) Very early indicators of response to systemic therapy in lymphoma patients based on alterations in water diffusivity—a preliminary experience in 20 patients undergoing whole-body diffusion-weighted imaging. Eur J Radiol 83:1655–1664
Furth C, Steffen IG, Amthauer H et al (2009) Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin's and adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma: analysis of a prospective multicenter trial. J Clin Oncol 27:4385–4391
Johnson P, Federico M, Kirkwood A et al (2016) Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med 374:2419–2429
Acknowledgements
AL was supported by a Cancer Research UK/ Engineering and Physical Sciences Research Council (CRUK/EPSRC) award (C1519/A10331 and C1519/A16463) from the University College London/King’s College London (UCL/KCL) Comprehensive Cancer Imaging Centre (CCIC).
This work was undertaken at the Comprehensive Biomedical Centre (BRC), University College Hospital London (UCLH), which received a proportion of the funding from the National Institute for Health Research (NIHR). The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health.
ST is an NIHR senior investigator.
The authors would like to acknowledge and thank University College London Cancer Trials Centre (UCL CTC), Dr Darren Edwards and Mrs K M Mak for their contribution towards this manuscript.
The trial was managed by the Cancer Research UK and University College London Cancer Trials Centre. The authors would also like to thank the patients and their families who took part in the study and the investigators and research staff at the participating centre.
Funding
This study has received funding from Cancer Research UK, project number CRUK ASC 12707.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Stuart A Taylor.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Andre Lopes) is a statistician with significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• prospective
• diagnostic or prognostic study/observational
• performed at one institution
Electronic supplementary material
ESM 1
(DOC 343 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Latifoltojar, A., Punwani, S., Lopes, A. et al. Whole-body MRI for staging and interim response monitoring in paediatric and adolescent Hodgkin’s lymphoma: a comparison with multi-modality reference standard including 18F-FDG-PET-CT. Eur Radiol 29, 202–212 (2019). https://doi.org/10.1007/s00330-018-5445-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5445-8