Abstract
Objective
To investigate the feasibility of highly efficient and controllable stem cell labelling for cellular MRI.
Methods
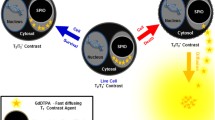
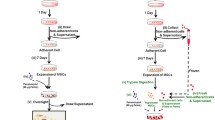
A new class of cationic, superparamagnetic iron oxide nanoparticle (SPION)-loaded nanovesicles was synthesised to label rat bone marrow mesenchymal stem cells without secondary transfection agents. The optimal labelling conditions and controllability were assessed, and the effect of labelling on cell viability, proliferation activity and multilineage differentiation was determined. In 18 rats, focal ischaemic cerebral injury was induced and the rats randomly injected with 1 × 106 cells labelled with 0-, 8- or 20-mV nanovesicles (n = 6 each). In vivo MRI was performed to follow grafted cells in contralateral striata, and results were correlated with histology.
Results
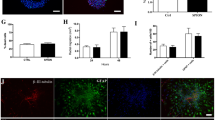
Optimal cell labelling conditions involved a concentration of 3.15 μg Fe/mL nanovesicles with 20-mV positive charge and 1-h incubation time. Labelling efficiency showed linear change with an increase in the electric potentials of nanovesicles. Labelling did not affect cell viability, proliferation activity or multilineage differentiation capacity. The distribution and migration of labelled cells could be detected by MRI. Histology confirmed that grafted cells retained the label and remained viable.
Conclusion
Stem cells can be effectively and safely labelled with cationic, SPION-loaded nanovesicles in a controllable way for cellular MRI.
Key Points
• Stem cells can be effectively labelled with cationic, SPION-loaded nanovesicles.
• Labelling did not affect cell viability, proliferation or differentiation.
• Cellular uptake of SPION could be controlled using cationic nanovesicles.
• Labelled cells could migrate along the corpus callosum towards cerebral infarction.
• The grafted, labelled cells retained the label and remained viable.








Similar content being viewed by others
References
Mimeault M, Hauke R, Batra SK (2007) Stem cells: a revolution in therapeutics—recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther 82:252–264
Mimeault M, Batra SK (2006) Concise review: recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells 24:2319–2345
Lee Z, Dennis JE, Gerson SL (2008) Imaging stem cell implant for cellular-based therapies. Exp Biol Med (Maywood) 233:930–940
Walczak P, Bulte JW (2007) The role of noninvasive cellular imaging in developing cell-based therapies for neurodegenerative disorders. Neurodegener Dis 4:306–313
Bulte JW (2009) In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol 193:314–325
Kraitchman DL, Gilson WD, Lorenz CH (2008) Stem cell therapy: MRI guidance and monitoring. J Magn Reson Imaging 27:299–310
Frank JA, Miller BR, Arbab AS et al (2003) Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology 228:480–487
Politi LS (2007) MR-based imaging of neural stem cells. Neuroradiology 49:523–534
Bulte JW, Douglas T, Witwer B et al (2001) Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol 19:1141–1147
Zhang C, Wangler B, Morgenstern B et al (2007) Silica- and alkoxysilane-coated ultrasmall superparamagnetic iron oxide particles: a promising tool to label cells for magnetic resonance imaging. Langmuir 23:1427–1434
Liu ZY, Wang Y, Liang CH et al (2009) In vitro labeling of mesenchymal stem cells with superparamagnetic iron oxide by means of microbubble-enhanced US exposure: initial experience. Radiology 253:153–159
Arbab AS, Yocum GT, Kalish H et al (2004) Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood 104:1217–1223
Bauer M, Kristensen BW, Meyer M et al (2006) Toxic effects of lipid-mediated gene transfer in ventral mesencephalic explant cultures. Basic Clin Pharmacol Toxicol 98:395–400
Dousset V, Tourdias T, Brochet T et al (2008) How to trace stem cells for MRI evaluation? J Neurol Sci 265:122–126
Ozpolat B, Sood AK, Lopez-Berestein G (2010) Nanomedicine based approaches for the delivery of siRNA in cancer. J Intern Med 267:44–53
Cai W, Chen X (2007) Nanoplatforms for targeted molecular imaging in living subjects. Small 3:1840–1854
Nagaya N, Fujii T, Iwase T et al (2004) Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol 287:H2670–6
Cao N, Cheng D, Zou S et al (2011) The synergistic effect of hierarchical assemblies of siRNA and chemotherapeutic drugs co-delivered into hepatic cancer cells. Biomaterials 32:2222–2232
Sun S, Zeng H, Robinson D et al (2004) Monodisperse MFe2O4(M=Fe, Co, Mn) nanoparticles. J Am Chem Soc 126:273–279
Arbab AS, Bashaw LA, Miller BR et al (2003) Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology 229:838–846
Kostura L, Kraitchman DL, Mackay AM et al (2004) Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed 17:513–517
Chen Y, Ito A, Takai K et al (2008) Blocking pterygopalatine arterial blood flow decreases infarct volume variability in a mouse model of intraluminal suture middle cerebral artery occlusion. J Neurosci Methods 174:18–24
Cook GM (1968) Glycoproteins in membranes. Biol Rev Camb Philos Soc 43:363–391
Angata T, Varki A (2002) Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem Rev 102:439–469
Daldrup-Link HE, Rudelius M, Oostendorp RA et al (2003) Targeting of hematopoietic progenitor cells with MR contrast agents. Radiology 228:760–767
Marcucci F, Lefoulon F (2004) Active targeting with particulate drug carriers in tumor therapy: fundamentals and recent progress. Drug Discov Today 9:219–228
Ma B, Hankenson KD, Dennis JE, Caplan AI, Goldstein SA, Kilbourn MR (2005) A simple method for stem cell labeling with fluorine 18. Nucl Med Biol 32:701–705
Gildehaus FJ, Haasters F, Drosse I et al (2011) Impact of indium-111 oxine labelling on viability of human mesenchymal stem cells in vitro, and 3D cell-tracking using SPECT/CT in vivo. Mol Imaging Biol 13:1204–1214
De Vries IJ, Lesterhuis WJ, Barentsz JO et al (2005) Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 23:1407–1413
Acknowledgements
This work is supported by the National Natural Science Foundation of China (grant number: 81071028, 50830107) and the Fundamental Research Funds for the Central Universities of China (grant number: 09ykpy04), and in part by the Guangdong Natural Science Foundation (grant number: 9151008901000001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, R.M., Cao, N., Zhang, F. et al. Controllable labelling of stem cells with a novel superparamagnetic iron oxide–loaded cationic nanovesicle for MR imaging. Eur Radiol 22, 2328–2337 (2012). https://doi.org/10.1007/s00330-012-2509-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2509-z