Abstract
Key message
PDE1 acts as a mediator of primary root growth in response to Pi deficiency.
Abstract
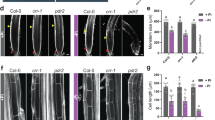
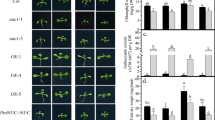
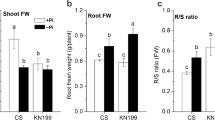
Phosphorus is commonly considered as a limiting nutrient for plant growth, which is mainly due to the immobility and uneven distribution of phosphate (Pi) in soils so that available Pi is not adequate in the rhizosphere. Although various mediators have been identified in Pi sensing and response, more details need to be uncovered in plant Pi-deficiency tolerance. Here, we isolated a mutant, termed pde1 (phosphate-deficiency sensitive 1), showing the hypersensitive Pi-deficiency-induced growth inhibition of primary roots. PDE1 encodes a hydroxyphenylpyruvate reductase with rare activity in vitro and repressed by Pi deficiency. Histochemical analysis displayed that Pi-deprived pde1 accumulated more Fe and reactive oxygen species (ROS) in primary roots than the wild type (WT). Addition of ferrozine, a Fe2+ chelator, or a ROS scavenger (e.g., thiourea and potassium iodide), alleviated the sensitivity of Pi-deficiency in pde1 primary roots. By contrast, pde1 showed reduced cotyledon expansion rate with treatment of H2O2 compared to WT. Taken together, these results suggested that PDE1 is responsible for regulating primary root growth in response to Pi deficiency, which is associated with ROS.






Similar content being viewed by others
Data availability
All relevant data are available from the corresponding author on request.
References
Balzergue C, Dartevelle T, Godon C, Laugier E, Meisrimler C, Teulon JM, Creff A, Bissler M, Brouchoud C, Hagège A, Müller J, Chiarenza S, Javot H, Becuwe-Linka N, David P, Péret B, Delannoy E, Thibaud MC, Armengaud J, Abel S, Pellequer JL, Nussaume L, Desnos T (2017) Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation. Nat Commun 8:15300
Bian L, Wang Y, Bai H, Li H, Zhang C, Chen J, Xu W (2021) Melatonin-ROS signal module regulates plant lateral root development. Plant Signal Behav 16:1901447
Chevalier F, Pata M, Nacry P, Doumas P, Rossignol M (2003) Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions. Plant, Cell Environ 26:1839–1850
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Crombez H, Motte H, Beeckman T (2019) Tackling plant phosphate starvation by the roots. Dev Cell 48:599–615
Dong J, Piñeros MA, Li X, Yang H, Liu Y, Murphy AS, Kochian LV, Liu D (2017) An Arabidopsis ABC transporter mediates phosphate deficiency-induced remodeling of root architecture by modulating iron homeostasis in roots. Mol Plant 10:244–259
Dunand C, Crèvecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174:332–341
Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Gutierrez-Alanis D, Ojeda-Rivera JO, Yong-Villalobos L, Cardenas-Torres L, Herrera-Estrella L (2018) Adaptation to phosphate scarcity: tips from Arabidopsis roots. Trends Plant Sci 23:721–730
Hirsch J, Marin E, Floriani M, Chiarenza S, Richaud P, Nussaume L, Thibaud MC (2006) Phosphate deficiency promotes modification of iron distribution in Arabidopsis plants. Biochimie 88:1767–1771
Hoehenwarter W, Monchgesang S, Neumann S, Majovsky P, Abel S, Muller J (2016) Comparative expression profiling reveals a role of the root apoplast in local phosphate response. BMC Plant Biol 16:106
Hoekenga OA, Maron LG, Piñeros MA, Cançado GM, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T, Matsumoto H, Yamamoto Y, Koyama H, Kochian LV (2006) AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci USA 103:9738–9743
Hücherig S, Petersen M (2013) RNAi suppression and overexpression studies of hydroxyphenylpyruvate reductase (HPPR) and rosmarinic acid synthase (RAS) genes related to rosmarinic acid biosynthesis in hairy root cultures of Coleus blumei. Plant Cell Tiss Organ Cult (PCTOC) 113:375–385
Kelner MJ, Bagnell R, Welch KJ (1990) Thioureas react with superoxide radicals to yield a sulfhydryl compound. Explanation for protective effect against paraquat. J Biol Chem 265:1306–1311
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O2⋅−, H2O2, and ⋅OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123; discussion 3001
Liu D (2021) Root developmental responses to phosphorus nutrition. J Integr Plant Biol 63:1065–1090
López-Bucio JS, Salmerón-Barrera GJ, Ravelo-Ortega G, Raya-González J, León P, de la Cruz HR, Campos-García J, López-Bucio J, Guevara-García ÁA (2019) Mitogen-activated protein kinase 6 integrates phosphate and iron responses for indeterminate root growth in Arabidopsis thaliana. Planta 250:1177–1189
Lynch JP, Brown KM (2001) Topsoil Foraging an—Architectural Adaptation of Plants to Low Phosphorus Availability. 237:225–237
Manzano C, Pallero-Baena M, Casimiro I, De Rybel B, Orman-Ligeza B, Van Isterdael G, Beeckman T, Draye X, Casero P, Del Pozo JC (2014) The emerging role of reactive oxygen species signaling during lateral root development. Plant Physiol 165:1105–1119
Meguro R, Asano Y, Odagiri S, Li C, Iwatsuki H, Shoumura K (2007) Nonheme-iron histochemistry for light and electron microscopy: a historical, theoretical and technical review. Arch Histol Cytol 70:1–19
Misson J, Raghothama KG, Jain A, Jouhet J, Block MA, Bligny R, Ortet P, Creff A, Somerville S, Rolland N, Doumas P, Nacry P, Herrerra-Estrella L, Nussaume L, Thibaud MC (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA 102:11934–11939
Mora-Macías J, Ojeda-Rivera JO, Gutiérrez-Alanís D, Yong-Villalobos L, Oropeza-Aburto A, Raya-González J, Jiménez-Domínguez G, Chávez-Calvillo G, Rellán-Álvarez R, Herrera-Estrella L (2017) Malate-dependent Fe accumulation is a critical checkpoint in the root developmental response to low phosphate. Proc Natl Acad Sci USA 114:E3563–E3572
Müller J, Toev T, Heisters M, Teller J, Moore KL, Hause G, Dinesh DC, Bürstenbinder K, Abel S (2015) Iron-dependent callose deposition adjusts root meristem maintenance to phosphate availability. Dev Cell 33:216–230
Nacry P, Canivenc G, Muller B, Azmi A, Van Onckelen H, Rossignol M, Doumas P (2005) A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol 138:2061–2074
Orman-Ligeza B, Parizot B, de Rycke R, Fernandez A, Himschoot E, Van Breusegem F, Bennett MJ, Périlleux C, Beeckman T, Draye X (2016) RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 143:3328–3339
Péret B, Clément M, Nussaume L, Desnos T (2011) Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci 16:442–450
Petersen M, Simmonds MS (2003) Rosmarinic acid. Phytochemistry 62:121–125
Prakash V, Vishwakarma K, Singh VP, Rai P, Ramawat N, Tripathi DK, Sharma S (2020) NO and ROS implications in the organization of root system architecture. Physiol Plant 168:473–489
Reyt G, Boudouf S, Boucherez J, Gaymard F, Briat JF (2015) Iron- and ferritin-dependent reactive oxygen species distribution: impact on Arabidopsis root system architecture. Mol Plant 8:439–453
Shin R, Schachtman DP (2004) Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci USA 101:8827–8832
Shin R, Berg RH, Schachtman DP (2005) Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol 46:1350–1357
Silva-Navas J, Conesa CM, Saez A, Navarro-Neila S, Garcia-Mina JM, Zamarreño AM, Baigorri R, Swarup R, Del Pozo JC (2019) Role of cis-zeatin in root responses to phosphate starvation. New Phytol 224:242–257
Simonzadeh N, Jaselskis B (1984) Reaction of iron(III) with tiron in the presence of ferrozine, and determination of tiron. Talanta 31:715–716
Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T (2007) Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet 39:792–796
Timm S, Nunes-Nesi A, Pärnik T, Morgenthal K, Wienkoop S, Keerberg O, Weckwerth W, Kleczkowski LA, Fernie AR, Bauwe H (2008) A cytosolic pathway for the conversion of hydroxypyruvate to glycerate during photorespiration in Arabidopsis. Plant Cell 20:2848–2859
Trócsányi E, György Z, Zámboriné-Németh É (2020) New insights into rosmarinic acid biosynthesis based on molecular studies. Curr Plant Biol 23:100162
Tsukagoshi H, Busch W, Benfey PN (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143:606–616
Wang J, Pan X, Han Y, Guo D, Guo Q, Li R (2012) Rosmarinic acid from eelgrass shows nematicidal and antibacterial activities against pine wood nematode and its carrying bacteria. Mar Drugs 10:2729–2740
Wang ZP, Xing HL, Dong L, Zhang HY, Han CY, Wang XC, Chen QJ (2015) Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol 16:144
Wang GQ, Chen JF, Yi B, Tan HX, Zhang L, Chen WS (2017) HPPR encodes the hydroxyphenylpyruvate reductase required for the biosynthesis of hydrophilic phenolic acids in Salvia miltiorrhiza. Chin J Nat Med 15:917–927
Wang X, Wang Z, Zheng Z, Dong J, Song L, Sui L, Nussaume L, Desnos T, Liu D (2019) Genetic dissection of Fe-dependent signaling in root developmental responses to phosphate deficiency. Plant Physiol 179:300–316
Wang Y, Dai X, Xu G, Dai Z, Chen P, Zhang T, Zhang H (2021) The Ca2+-CaM signaling pathway mediates potassium uptake by regulating reactive oxygen species homeostasis in tobacco roots under low-K+ Stress. Front Plant Sci 12:658609
Williamson LC, Ribrioux SP, Fitter AH, Leyser HM (2001) Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol 126:875–882
Xu JJ, Fang X, Li CY, Zhao Q, Martin C, Chen XY, Yang L (2018) Characterization of Arabidopsis thaliana hydroxyphenylpyruvate reductases in the tyrosine conversion pathway. Front Plant Sci 9:1305
Xu JM, Wang ZQ, Wang JY, Li PF, Jin JF, Chen WW, Fan W, Kochian LV, Zheng SJ, Yang JL (2020) Low phosphate represses histone deacetylase complex1 to regulate root system architecture remodeling in Arabidopsis. New Phytol 225:1732–1745
Zheng Z, Wang Z, Wang X, Liu D (2019) Blue light-triggered chemical reactions underlie phosphate deficiency-induced inhibition of root elongation of Arabidopsis seedlings grown in petri dishes. Mol Plant 12:1515–1523
Zheng M, Zhu C, Yang T, Qian J, Hsu YF (2020) GSM2, a transaldolase, contributes to reactive oxygen species homeostasis in Arabidopsis. Plant Mol Biol 104:39–53
Zhou Z, Li J, Zhu C, Jing B, Shi K, Yu J, Hu Z (2022) Exogenous rosmarinic acid application enhances thermotolerance in tomatoes. Plants 11:1172
Funding
This work was supported by the Chongqing science and technology forestry project (YB 2023–4) and the Chongqing Municipal Education Commission for postgraduates innovation program (CYB21101).
Author information
Authors and Affiliations
Contributions
MZ and YFH designed the research. LYW, ML, HZ and XY performed the experiments. JQ and LYW analyzed the data. LYW, JQ, MZ and YFH wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Li Tian.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Qian, J., Li, M. et al. Arabidopsis PDE1 confers phosphate-deficiency tolerance in primary root growth. Plant Cell Rep 43, 8 (2024). https://doi.org/10.1007/s00299-023-03120-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00299-023-03120-8