Abstract
Key messages
Cu/Zn SOD and other genes may be critical indicators of a stress response to reactive oxygen species (ROS) accumulation in 48 h germinated rice embryos subjected to vitrification cryopreservation.
Abstract
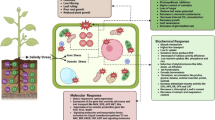
In the current study, reactive oxygen species (ROS) accumulation was investigated in 48 h germinated rice embryos during the vitrification–cryopreservation process. We found that vitrification–cryopreservation significantly affected ROS levels, especially superoxide anion levels, in 48 h germinated rice embryos. Malonaldehyde content in the apical meristems of germinated embryos was significantly positively correlated with the rate of superoxide anion generation and the highest levels of malonaldehyde content were reached after vitrification treatment. Cell viability in 48 h germinated embryos was significantly negatively correlated with the rate of superoxide anion generation, malonaldehyde content, and electrolyte leakage. Spatial and temporal patterns in ROS accumulation in these embryos existed during the vitrification procedure. Among the vitrification–cryopreservation treatments we assessed, the preculture treatment was found to stimulate superoxide anion generation and to activate the response system in the apical meristems of germinated embryos. Loading treatments motivated the catalase and ascorbate peroxidase activities. During the vitrification–dehydration treatment, oxidative stress reached the highest levels causing an antioxidative response. This response involved antioxidant enzymes promoting detoxification of ROS. Based on a comprehensive correlation analysis involving ROS accumulation, cell viability, the activities of antioxidant enzymes, and gene expression profiles, Cu/Zn SOD, CAT1, APX7, GR2, GR3, MDHAR1, and DHAR1 may be critical indicators of oxidative stress affected by the vitrification–cryopreservation treatments. The investigation of these antioxidative responses in 48 h germinated rice embryos may, therefore, provide useful information with respect to plant vitrification–cryopreservation.








Similar content being viewed by others
Abbreviations
- ·OH:
-
Hydroxyl radicals
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbic acid
- CAT:
-
Catalase
- DAB:
-
Diaminobenzidine
- DHA:
-
Docosahexaenoic acid
- DHAR:
-
Dehydroascorbate reductase
- DW:
-
Dry weight
- EDTA:
-
Ethylene diamine tetraacetic acid
- EL:
-
Electrolyte leakage
- FW:
-
Fresh weight
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione
- GSSG:
-
Oxidized glutathione
- H2O2 :
-
Hydrogen peroxide
- HPLC:
-
High performance liquid chromatography
- LN:
-
Liquid nitrogen
- LS:
-
Loading solution
- MDA:
-
Malonaldehyde
- MDHAR:
-
Monodehydroascorbate reductase
- MS:
-
Murashige and Skoog
- NBT:
-
Nitroblue tetrazolium
- O2 ·− :
-
Superoxide anion
- PVS:
-
Plant vitrification solution
- qRT-PCR:
-
Quantitative reverse-transcription polymerase chain reactions
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TTC:
-
Triphenyltetrazolium chloride
References
Alscher R, Erturk N, Heath L (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53(372):1331
Arrigoni O, De GL, Tommasi F, Liso R (1992) Changes in the ascorbate system during seed development of Vicia faba L. Plant Physiol 99(1):235
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition. Elsevier, Amsterdam, pp 228–287
Beatty S, Koh HH, Phil M, Henson D, Boulton M (2000) The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 45(2):115
Benson EE (2008) Cryopreservation of phytodiversity: a critical appraisal of theory and practice. Crit Rev Plant Sci 27(3):141–219
Benson E, Bremner D (2004) Oxidative stress in the frozen plant: a free radical point of view. In: Life in the frozen state. CRC Press, London, pp 205–242
Bergmeyer HU (1963) Methods of enzymatic analysis. Academic Press, New York, pp 2253–2259
Berjak P, Pammenter NW (2013) Implications of the lack of desiccation tolerance in recalcitrant seeds. Front Plant Sci 4(2):478
Berjak P, Varghese B, Pammenter NW (2011) Cathodic amelioration of the adverse effects of oxidative stress accompanying procedures necessary for cryopreservation of embryonic axes of recalcitrant-seeded species. Seed Sci Res 21:187–203
Beyer WF Jr, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161(2):559–566
Bhoomika K, Pyngrope S, Dubey RS (2013) Differential responses of antioxidant enzymes to aluminum toxicity in two rice (Oryza sativa L.) cultivars with marked presence and elevated activity of Fe SOD and enhanced activities of Mn SOD and catalase in aluminum tolerant cultivar. Plant Growth Regul 71(3):235–252
Bowler C, Slooten L, Vandenbranden S, De RR, Botterman J, Sybesma C, Van MM, Inzé D (1991) Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. Embo J 10(7):1723–1732
Bowler C, And MVM, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Biol 43(1):83–116
Chen GQ, Ren L, Zhang J, Reed BM, Zhang D, Shen XH (2015) Cryopreservation affects ROS-induced oxidative stress and antioxidant response in Arabidopsis seedlings. Cryobiology 70(1):38–47
Chen GQ, Ren L, Zhang D, Shen XH (2016) Glutathione improves survival of cryopreserved embryogenic calli of Agapanthus praecox subsp. orientalis. Acta Physiol Plant 38(10):250
D’Autréaux B, Toledano MB (2007) Ros as signalling molecules: mechanisms that generate specificity in ros homeostasis. Nat Rev Mol Cell Biol 8(10):813–824
Dalton DA, Langeberg L, Treneman NC (1993) Correlations between the ascorbate-glutathione pathway and effectiveness in legume root nodules. Physiol Plant 87(3):365–370
Deng WK, Wang YB, Liu ZX, Cheng H, Xue Y (2014)HemI: a toolkit for illustrating heatmaps. PLoS One. https://doi.org/10.1371/journal.pone.0111988
Deng Z, Zhao M, Liu H, Wang Y, Li D (2015) Molecular cloning, expression profiles and characterization of a glutathione reductase in Hevea brasiliensis. Plant Physiol Biochem 96:53–63
Driever SM, Fryer MJ, Mullineaux PM, Baker NR (2009) Imaging of reactive oxygen species in vivo. Methods Mol Biol 479:109
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70(2):616–620
Engelmann F (1997) Importance of desiccation for the cryopreservation of recalcitrant seed and vegetatively propagated species. Plant Genet Resour Newslett 112:9–18
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In vitro Cell Dev Biol Plant 47(1):5–16
Engelmann F, Arnao MTG, Wu Y, Escobar R (2008) Development of encapsulation dehydration. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, New York, pp 59–75
Fang JY, Wetten A, Johnston J (2008) Headspace volatile markers for sensitivity of cocoa (Theobroma cacao L.) somatic embryos to cryopreservation. Plant Cell Rep 27(3):453
Freitas RTD, Paiva R, Sales TS, Silva DPCD, Reis MVD, Souza ACD (2016) Cryopreservation of Coffea arabica L. Zygotic Embryos by Vitrification. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 44(4):445
Gaff DF, Okong’O-Ogola O (1971) The Use of non-permeating pigments for testing the survival of cells. J Exp Bot 22(72):756–758
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Guo Z, Ou W, Lu S, Zhong Q (2006) Differential responses of antioxidative system to chilling and drought in fourrice cultivars differing in sensitivity. Plant Physiol Biochem 44(11–12):828–836
Halder T, Upadhyaya G, Basak C, Das A, Chakraborty C, Ray S (2018) Dehydrins impart protection against oxidative stress in transgenic tobacco plants. Front Plant Sci 9:136
Halliwell B, Gutteridge JMC (1985) Free radicals in biology and medicine. J Free Radic Biol Med 1(4):331–332
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. Cryo Lett 25(1):3–22
Harding K, Johnston JW, Benson EE (2008) Concepts in cryobionomics: a case study of Ribes genotype responses to cryopreservation in relation to thermal analysis oxidative stress nucleic acid methylation and transcriptional activity. In: Laamanen J, Uosukainen M, Häggman H, Nukari A, Rantala S (eds) cryopreservation of crop species in Europe, proceedings of CRYOPLANET COST Action 871, 20–23 February 2008, Oulu, MTT Agrifood Research Working Papers 153
He YQ, Cheng JP, Li XD, Zeng P, Yang B, Zhang HS, Wang ZF (2016) Acquisition of desiccation tolerance during seed development isassociated with oxidative processes in rice. Botany 94(2):91–101
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Hong CY, Kao CH (2007) Expression of ASCORBATE PEROXIDASE 8 in roots of rice (Oryza sativa L.) seedlings in response to NaCl. J Exp Bot 58(12):3273
Hong CY, Chao YY, Yang MY, Cheng SY, Cho SC, Kao CH (2009) NaCl-induced expression of glutathione reductase in roots of rice (Oryza sativa L.) seedlings is mediated through hydrogen peroxide but not abscisic acid. Plant Soil 320(1/2):103–115
Johnston JW, Harding K, Benson EE (2007) Antioxidant status and genotypic tolerance of Ribes in vitro cultures to cryopreservation. Plant Sci 172(3):524–534
Kaczmarczyk A, Funnekotter B, Menon A, Phang PY, Al-Hanbali A, Bunn E, Mancera RL (2012) Current issues in plant cryopreservation. In: Katkov II (ed) Current frontiers in cryobiology. InTech, Rijeka
Kim DW, Rakwal R, Agrawal GK, Jung YH, Shibato J, Jwa NS, Iwahashi Y, Iwahashi H, Kim DH, Shim IeS, Usui K (2005) A hydroponic rice seedling culture model system for investigating proteome of salt stress in rice leaf. Electrophoresis 26(23):4521
Kordrostami M, Rabiei B, Kumleh HH (2017) Different physiobiochemical and transcriptomic reactions of rice (Oryza sativa L.) cultivars differing in terms of salt sensitivity under salinity stress. Environ Sci Pollut Res 24(8):7184
Kovalchuk I, Turdiev T, Mukhitdinova Z, Frolov S, Reed BM, Kairova G (2014) New techniques for rapid cryopreservation of dormant vegetative buds. Acta Hort 1039(1039):137–146
Lee S, Chung MS, Ji EK, Lee GW, Jeong YS, Min HL, Hong SH, Lee SS, Kim JH, Chung BY (2015) Liquid chromatography-tandem mass spectrometry-assisted identification of two salinity-inducible ascorbate peroxidases in a salt-sensitive rice cultivar (Oryza sativa L. cv. ‘IR-29). Plant Growth Regul 75(1):143–153
Li HY, Luo HJ, Li DY, Hu T, Fu JM (2012) Antioxidant enzyme activity and gene expression in response to lead stress in perennial ryegrass. J Am Soc Hortic Sci 137(2):80–85
Li X, Cai J, Liu F, Dai T, Cao W, Jiang D (2014) Cold priming drives the sub-cellular antioxidant systems to protect photosynthetic electron transport against subsequent low temperature stress in winter wheat. Plant Physiol Biochem 82(3):34–43
Liu M, Liu X, Li M, Fang M, Chi W (2010) Neural-network model for estimating leaf chlorophyll concentration in rice under stress from heavy metals using four spectral indices. Biosys Eng 106(3):223–233
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265
Lynch PT, Siddika A, Johnston JW, Trigwell SM, Mehra A, Benelli C, Lambardi M, Benson EE (2011) Effects of osmotic pretreatments on oxidative stress, antioxidant profiles and cryopreservation of olive somatic embryos. Plant Sci 181(1):47–56
Lyu SR, Wu WT, Hou CC, Hsieh WH (2010) Study of cryopreservation of articular chondrocytes using the Taguchi method. Cryobiology 60(2):165–176
Madamanchi NR, Alscher RG (1991) Metabolic bases for differences in sensitivity of two pea cultivars to sulfur dioxide. Plant Physiol 97(1):88–93
Matsubara C, Nishikawa Y, Yoshida Y, Takamura K (1983) A spectrophotometric method for the determination of free fatty acid in serum using acyl-coenzyme A synthetase and acyl-coenzyme A oxidase. Anal Biochem 130(1):128–133
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Mostofa MG, Hossain MA, Fujita M (2015) Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma 252(2):461–475
Murashige T, Skoog F (2010) Murashige and Skoog medium. Alphascript Publishing, Saarbrücken
Nakano Y, Asada K (1981) Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol 22(5):867–880
Nguyen HM, Sako K, Matsui A, Suzuki Y, Mostofa MG, Ha CV, Tanaka M, Tran LSP, Habu Y, Seki M (2017) Ethanol enhances high-salinity stress tolerance by detoxifying reactive oxygen species in Arabidopsis thaliana and rice. Front Plant Sci 8:1–10
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91(1):67–73
Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquezgarcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35(2):454–484
Orozco-Cárdenas ML, Narváezvásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13(1):179–191
Pritchard HW (2007) Cryopreservation of desiccation-tolerant seeds. Methods Mol Biol 368:185–201
Rao MV, Davis KR (1999) Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J Cell Mol Biol 17(6):603–614
Ren L, Zhang D, Jiang XN, Gai Y, Wang WM, Reed BM, Shen XH (2013) Peroxidation due to cryoprotectant treatment is a vital factor for cell survival in Arabidopsis cryopreservation. Plant Sci 212(3):37
Rogulska O, Petrenko Y, Petrenko A (2017) DMSO-free cryopreservation of adipose-derived mesenchymal stromal cells: expansion medium affects post-thaw survival. Cytotechnology 69(2):265–276
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Sershen VB, Pammenter NW, Berjak P (2012) Cryo-tolerance of zygotic embryos from recalcitrant seeds in relation to oxidative stress—a case study on two amaryllid species. J Plant Physiol 169(10):999
Sewelam N, Kazan K, Schenk PM (2016) Global plant stress signaling: reactive oxygen species at the cross-road. Front Plant Sci 7(187):187
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:217037
Sharma I, Bhardwaj R, Pati PK (2015) Exogenous application of 28-homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety Pusa Basmati-1. J Plant Growth Regul 34(3):509–518
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82(2):291–295
Skyba M, Urbanová M, Kapchina-Toteva V, Košuth J, Harding K, Čellárová E (2010) Physiological, biochemical and molecular characteristics of cryopreserved Hypericum perforatum L. shoot tips. CryoLetters 31(3):249–260
Skyba M, Petijová L, Košuth J, Koleva DP, Ganeva TG, Kapchina-Toteva VM, Čellárová E (2012) Oxidative stress and antioxidant response in Hypericum perforatum L. plants subjected to low temperature treatment. J Plant Physiol 169(10):955
Subbarayan K, Rolletschek H, Senula A, Ulagappan K, Hajirezaei MR, Keller ERJ (2015) Influence of oxygen deficiency and the role of specific amino acids in cryopreservation of garlic shoot tips. BMC Biotechnol 15(1):40
Teixeira FK, Menezesbenavente L, Galvão VC, Margis R, Margispinheiro M (2006) Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments. Planta 224(2):300–314
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J 11(6):1187–1194
Towill LE, Mazur P (1975) Studies on the reduction of 2,3,5-triphenyltetrazolium chloride as a viability assay for plant tissue cultures. Can J Bot 53(11):1097–1102
Vighi IL, Benitez LC, Amaral MN, Moraes GP, Auler PA, Rodrigues GS, Deuner S, Maia LC, Braga EJB (2017) Functional characterization of the antioxidant enzymes in rice plants exposed to salinity stress. Biol Plant 61(3):540–550
Wang ZF, Wang JF, Wang FH, Bao YM, Wu YY, Zhang HS (2010) Segregation analysis of rice seed germination under cold stress using major gene plus polygene mixed inheritance model. Seed Sci Technol 38(1):104–113
Wang Y, Zhang L, Zhang L, Xing T, Peng J, Sun S, Chen G, Wang X (2013) A novel stress-associated protein SbSAP14 from Sorghum bicolor confers tolerance to salt stress in transgenic rice. Mol Breed 32(2):437–449
Wang B, Li JW, Zhang ZB, Wang RR, Ma YL, Blystad DR, Keller ERJ, Wang QC (2014a) Three vitrification-based cryopreservation procedures cause different cryo-injuries to potato shoot tips while all maintain genetic integrity in regenerants. J Biotechnol 184:47–55
Wang B, Wang RR, Cui ZH, Bi WL, Li JW, Li BQ, Ozudogru EA, Volk GM, Wang QC (2014b) Potential applications of cryogenic technologies to plant genetic improvement and pathogen eradication. Biotechnol Adv 32(3):583–595
Wang P, Shuangen YU, Zhang C (2016) Morgan model of rice under alternating stress of drought and waterlogging. J Irrig Drain 35(5):62–66 (in Chinese)
Wang W, Zhang X, Deng F, Yuan R, Shen F (2017) Genome-wide characterization and expression analyses of superoxide dismutase (SOD) genes in Gossypium hirsutum. BMC Genom 18(1):376
Weindruch R (1996) Caloric restriction and aging. Sci Am 274(1):46–52
Wen B, Wang R, Cheng H, Song S (2010) Cytological and physiological changes in orthodox maize embryos during cryopreservation. Protoplasma 239(4):57–67
Wen B, Cai C, Wang R, Song S, Song J (2012) Cytological and physiological changes in recalcitrant Chinese fan palm (Livistona chinensis) embryos during cryopreservation. Protoplasma 249(2):323
Whitaker C, Beckett RP, Minibayeva FV, Kranner I (2010) Production of reactive oxygen species in excised, desiccated and cryopreserved explants of Trichilia dregeana Sond. South Afr J Bot 76(1):112–118
Willekens H, Langebartels C, Tire C, Montagu MV, Inze D, Camp WV (1994) Differential expression of catalase genes in Nicotiana plumbaginifolia (L.). Proc Natl Acad Sci USA 91(22):10450–10454
Wu TM, Lin WR, Kao YT, Hsu YT, Yeh CH, Hong CY, Kao CH (2013) Identification and characterization of a novel chloroplast/mitochondria co-localized glutathione reductase 3 involved in salt stress response in rice. Plant Mol Biol 83(4–5):379
Yin L, Wang SW, Eltayeb AE, Uddin MI, Yamamoto Y, Tsuji W, Takeuchi Y, Tanaka K (2010) Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231(3):609
Yin GK, Xin X, Song C, Chen XL, Zhang JM, Wu SH, Li R, Liu X, Lu XX (2014) Activity levels and expression of antioxidant enzymes in the ascorbate-glutathione cycle in artificially aged rice seed. Plant Physiol Biochem 80:1–9
Young TE, Gallie DR, Demason DA (1997) Ethylene-mediated programmed cell death during maize endosperm development of wild-type and shrunken2 genotypes. Plant Physiol 115(2):737
Zhang D, Ren L, Chen GQ, Zhang J, Reed BM, Shen XH (2015) ROS-induced oxidative stress and apoptosis-like event directly affect the cell viability of cryopreserved embryogenic callus in Agapanthus praecox. Plant Cell Rep 34(9):1499
Zhang J, Luo W, Zhao Y, Xu Y, Song S, Chong K (2016) Comparative metabolomic analysis reveals a reactive oxygen species-dominated dynamic model underlying chilling environment adaptation and tolerance in rice. New Phytol 211:1295–1310
Acknowledgements
This study was supported by Agricultural Science and Technology Innovation Program/Crop Germplasm resources Preservation and sharing Innovation Team (CAAS, ASTIP), Crop Germplasm Resources Protection, Utilization Special Grant from the Ministry of Agriculture, and Science and Technology Innovation Grant from Fujian Agriculture and Forestry University (KFA17414A). The authors would like to acknowledge Gayle M Volk (National Center for Genetic Resources Preservation, United States Department of Agriculture, Fort Collins, Colorado, U.S.A.) for assistance in revisions of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Qiaochun Wang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, B., Zhang, JM., Chen, XL. et al. Oxidative damage and antioxidative indicators in 48 h germinated rice embryos during the vitrification–cryopreservation procedure. Plant Cell Rep 37, 1325–1342 (2018). https://doi.org/10.1007/s00299-018-2315-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-018-2315-4