Abstract
Key message
Ectopic expression of auxin glycosyltransferase UGT84A2 in Arabidopsis can delay flowering through increased indole-3-butyric acid and suppressed transcription of ARF6, ARF8 and flowering-related genes FT, SOC1, AP1 and LFY.
Abstract
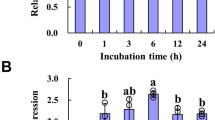
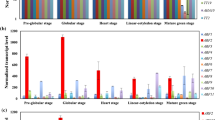
Auxins are critical regulators for plant growth and developmental processes. Auxin homeostasis is thus an important issue for plant biology. Here, we identified an indole-3-butyric acid (IBA)-specific glycosyltransferase, UGT84A2, and characterized its role in Arabidopsis flowering development. UGT84A2 could catalyze the glycosylation of IBA, but not indole-3-acetic acid (IAA). UGT84A2 transcription expression was clearly induced by IBA. When ectopically expressing in Arabidopsis, UGT84A2 caused obvious delay in flowering. Correspondingly, the increase of IBA level, the down-regulation of AUXIN RESPONSE FACTOR 6 (ARF6) and ARF8, and the down-regulation of flowering-related genes such as FLOWERING LOCUS T (FT), SUPPRESSOR OF OVEREXPRESSION OF CO1(SOC1), APETALA1 (AP1), and LEAFY(LFY) were observed in transgenic plants. When exogenously applying IBA to wild-type plants, the late flowering phenotype, the down-regulation of ARF6, ARF8 and flowering-related genes recurred. We examined the arf6arf8 double mutants and found that the expression of flowering-related genes was also substantially decreased in these mutants. Together, our results suggest that glycosyltransferase UGT84A2 may be involved in flowering regulation through indole-3-butyric acid-mediated transcriptional repression of ARF6, ARF8 and downstream flowering pathway genes.







Similar content being viewed by others
References
Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309:1052–1056
Achard P, Herr A, Baulcombe DC, Harberd NP (2004) Modulation of floral development by a gibberellin-regulated microRNA. Development 131:3357–3365
Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C (2004) Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427:164–167
Campbell JA, Davies GJ, Bulone V, Henrissat B (1997) A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem J 326:929–939
Cho LH, Yoon J, An G (2016) The control of flowering time by environmental factors. Plant J. doi: 10.1111/tpj.13461
Chong J, Baltz R, Schmitt C, Beffa R, Fritig B, Saindrenan P (2002) Downregulation of a pathogen-responsive tobacco UDP-Glc:phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. Plant Cell 14:1093–1107
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743
Corbesier L, Vincent C, Jang S, Fornara F, Fan QZ, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316:1030–1033
Gachon CM, Langlois-Meurinne M, Saindrenan P (2005) Plant secondary metabolism glycosyltransferases: the emerging functional analysis. Trends Plant Sci 10:542–549
Gendall AR, Levy YY, Wilson A, Dean C (2001) The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell 107:525–535
Hou BK, Lim EK, Higgins G, Bowles DJ (2004) N-Glucosylation of cytokinins by glycosyltransferases of Arabidopsis thaliana. J Biol Chem 279:47822–47832
Jackson RG, Lim EK, Li Y, Kowalczyk M, Sandberg G, Hoggett J, Ashford DA, Bowles DJ (2001) Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J Biol Chem 276:4350–4356
Jackson RG, Kowalczyk M, Li Y, Higgins G, Ross J, Sandberg G, Bowles DJ (2002) Overexpression of an Arabidopsis gene encoding a glucosyltransferase of indole-3-acetic acid: phenotypic characterization of transgenic lines. Plant J 32:573–583
Jin SH, Ma XM, Han P, Wang B, Sun YG, Zhang GZ, Li YJ, Hou BK (2013) UGT74D1 is a novel auxin glycosyltransferase from Arabidopsis thaliana. PLoS One 8:e61705
Li L, Li X, Liu Y, Liu H (2016) Flowering responses to light and temperature. Sci China Life Sci 59:403–408
Liu X, Barkawi L, Gardner G, Cohen JD (2012) Transport of indole-3-butyric acid and indole-3-acetic acid in Arabidopsis thaliana hypocotyls using stable isotope labeling. Plant Physiol 158:1988–2000
Ludwig-Müller J (2000) Indole-3-butyric acid in plant growth and development. Plant Growth Regul 32:219–230
Ludwig-Müller J, Sass S, Sutter EG, Wodner M, Epstein E (1993) Indole-3-butyric acid in Arabidopsis thaliana. Identification and quantification. Plant Growth Regul 13:179–187
Ludwig-Müller J, Walz A, Slovin JP, Epstein E, Cohen JD, Dong WQ, Town CD (2005) Overexpression of Maize IAGLU in Arabidopsis thaliana alters plant growth and sensitivity to IAA but not IBA and 2,4-D. Plant Growth Regul 24:127–141
Monte E, Alonso JM, Ecker JR, Zhang YL, Li X, Young J, Austin-Phillips S, Quail PH (2003) Isolation and characterization of phyC mutants in Arabidopsis reveals complex crosstalk between phytochrome signaling pathways. Plant Cell 15:1962–1980
Murase K, Hirano Y, Sun TP, Hakoshima T (2008) Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 456:459–463
Ross J, Li Y, Lim E, Bowles DJ (2001) Higher plant glycosyltransferases. Genome Biol 2:REVIEWS3004
Scarpella E, Barkoulas M, Tsiantis M (2010) Control of leaf and vein development by auxin. Cold Spring Harb Perspect Biol 2:a001511
Sinlapadech T, Stout J, Ruegger MO, Deak M, Chapple C (2007) The hyper-fluorescent trichome phenotype of the brt1 mutant of Arabidopsis is the result of a defect in a sinapic acid:UDPG glucosyltransferase. Plant J 49:655–668
Siriwardana NS, Lamb RS (2012) The poetry of reproduction: the role of LEAFY in Arabidopsis thaliana flower formation. Int J Dev Biol 56:207–221
Srikanth A, Schmid M (2011) Regulation of flowering time: all roads lead to Rome. Cell Mol Life Sci 68:2013–2037
Strader LC, BarteL B (2009) The Arabidopsis PLEIOTROPIC DRUG RESISTANCE8/ABCG36 ATP binding cassette transporter modulates sensitivity to the auxin precursor indole-3-butyric acid. Plant Cell 21:1992–2007
Strader LC, Culler AH, Cohen JD, Bartel B (2010) Conversion of endogenous indole-3-butyric acid to indole-3-acetic acid drives cell expansion in Arabidopsis seedlings. Plant Physiol 153:1577–1586
Strader LC, Wheeler DL, Christensen SE, Berens JC, Cohen JD, Rampey RA, Bartel B (2011) Multiple facets of Arabidopsis seedling development require indole-3-butyric acid-derived auxin. Plant Cell 23:984–999
Sun XH, Ouyang Y, Chu JF, Yan J, Yu Y, Li XQ, Yang J, Yan CY (2014) An in-advance stable isotope labeling strategy for relative analysis of multiple acidic plant hormones in sub-milligram Arabidopsis thaliana seedling and a single seed. J Chromatogr A 1338: 67–76
Tognetti VB, Aken O, Morreel K, Vandenbroucke K, Cotte B, Clercq I, Chiwocha S, Fenske R, Prinsen E, Boerjan W, Genty B, Stubbs KA, Inze D, Breusegem F (2010) Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 22:2660–2679
Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136:1005–1016
Wang JW (2014) Regulation of flowering time by the miR156-mediated age pathway. J Exp Bot 65:4723–4730
Weis M, Lim EK, Bruce NC, Bowles DJ (2008) Engineering and kineticcharacterisation of two glucosyltransferases from Arabidopsis thaliana. Biochimie 90:830–834
Winter CM, Yamaguchi N, Wu MF, Wagner D (2015) Transcriptional programs regulated by both LEAFY and APETALA1 at the time of flower formation. Plant Physiol 155:55–73
Yamaguchi A, Abe M (2012) Regulation of reproductive development by non-coding RNA in Arabidopsis: to flower or not to flower. J Plant Res 125:693–704
Zhang GZ, Jin SH, Jiang XY, Dong RR, Li P, Li YJ, Hou BK (2016) Ectopic expression of UGT75D1, a glycosyltransferase preferring indole-3-butyric acid, modulates cotyledon development and stress tolerance in seed germination of Arabidopsis thaliana. Plant Mol Biol 90:77–93
Zolman B, Yoder A, Bartel B (2000) Genetic analysis of indole-3-butyric acid response in Arabidopsis thaliana reveals four mutant classes. Genetics 156:1323–1327
Acknowledgements
This research was supported by the National Natural Science Foundation of China (nos. 91217301 and 31570299 to BKH; no. 31500230 to SHJ) and by Shandong Provincial Natural Science Foundation of China (no. ZR2017PC007 to GZZ). We thank Dr. Jason W. Reed (Department of Biology, University of North Carolina) for kindly providing us with the arf6arf8 double mutant seeds. brt1-1 mutant was ordered from the European Arabidopsis Stock Centre (NASC). We thank Dr. Jinfang Chu (The Plant Hormone Platform, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for help with the measurement of IBA level.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Prakash P. Kumar.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, GZ., Jin, SH., Li, P. et al. Ectopic expression of UGT84A2 delayed flowering by indole-3-butyric acid-mediated transcriptional repression of ARF6 and ARF8 genes in Arabidopsis. Plant Cell Rep 36, 1995–2006 (2017). https://doi.org/10.1007/s00299-017-2225-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2225-x