Abstract
Key message
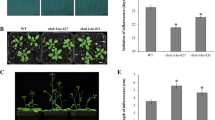
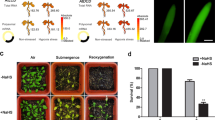
Both AtrbohD and AtrbohF promote the increases in activities of ADH, PDC, LDH, and Ca2+ levels, and induce the expression of multiple hypoxia response genes, thus improving Arabidopsis adaptation to oxygen deficiency.
Abstract
NADPH oxidase AtrbohD and AtrbohF cooperatively play key roles in regulation of growth and stress signaling in Arabidopsis. However, reports on AtrbohD and AtrbohF functioning together in hypoxia signaling are scarce, and the underlying mechanisms remain elusive. Here, we show that the double null mutant atrbohD/F is more sensitive to oxygen deprivation compared with wild type (WT) and the single mutant atrbohD and atrbohF. Under oxygen deficiency, enhancements of the transcripts of alcohol dehydrogenase 1 (ADH1) and pyruvate decarboxylase 1 (PDC1) and the activities of ADH, PDC and lactate dehydrogenase in WT are clearly reduced in the single mutants, and more strongly reduced in the double mutant. Moreover, increases in the production of ATP, H2O2 and Ca2+ in WT are significantly arrested in atrbohD, atrbohF, and especially in atrbohD/F. Hypoxia-promoted rise in the expression of some hypoxic responsive genes is also inhibited in atrbohD/F relative to WT, atrbohD and atrbohF. These genes include ethylene response factor 73, lactate dehydrogenase, MYB transcription factor 2, sucrose synthase 1 (SUS1), SUS4, heat stress transcription factor A2 and heat-shock protein 18.2. These results suggest that both AtrbohD and AtrbohF are essential for mediating hypoxia signaling. H2O2 derived from AtrbohD and AtrbohF triggers the Ca2+ increase and induces the expression of multiple hypoxia response genes, thus improving Arabidopsis tolerance to low-oxygen stress. These findings provide new insights into the mechanisms of AtrbohF in regulating the responses to oxygen deprivation in Arabidopsis.







Similar content being viewed by others
References
Bailey-Serres J, Chang R (2005) Sensing and signaling in response to oxygen deprivation in plants and other organisms. Ann Bot 96:507–518
Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296:2026–2028
Bieniawska Z, Paul Barratt DH, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM (2007) Analysis of the sucrose synthase gene family in Arabidopsis. Plant J 49:810–828
Chang R, Jang CJ, Branco-Price C, Nghiem P, Bailey-Serres J (2012) Transient MPK6 activation in response to oxygen deprivation and reoxygenation is mediated by mitochondria and aids seedling survival in Arabidopsis. Plant Mol Biol 78:109–122
Chang YL, Li WY, Miao H, Yang SQ, Li R, Wang X, Li WQ, Chen KM (2016) Comprehensive genomic analysis and expression profiling of the NOX gene families under abiotic stresses and hormones in plants. Genome Biol Evol 8:791–810
Chen L, Liao B, Qi H, Xie LJ, Huang L, Tan WJ, Zhai N, Yuan LB, Zhou Y, Yu LJ, Chen QF, Shu WS, Xiao S (2015) Autophagy contributes to regulation of the hypoxia response during submergence in Arabidopsis thaliana. Autophagy 11:2233–2246
Fukao T, Bailey-Serres J (2004) Plant responses to hypoxia. Is survival a balancing act? Trends Plant Sci 9:1403–1409
Gasch P, Fundinger M, Müller JT, Lee T, Bailey-Serres J, Mustroph A (2016) Redundant ERF-VII transcription factors bind to an evolutionarily conserved cis-motif to regulate hypoxia-responsive gene expression in Arabidopsis. Plant Cell 28:160–180
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30:1–47
Gibbs DJ, Lee SC, Isa NM, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey-Serres J, Holdsworth MJ (2011) Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479:415–418
Gibbs DJ, Conde JV, Berckhan S, Prasad G, Mendiondo GM, Holdsworth MJ (2015) Group VII ethylene response factors coordinate oxygen and nitric oxide signal transduction and stress responses in plants. Plant Physiol 169:23–31
Gonzali S, Loreti E, Cardarelli F, Novi G, Parlanti S, Pucciariello C, Bassolino L, Banti V, Licausi F, Perata P (2015) Universal stress protein HRU1 mediates ROS homeostasis under anoxia. Nat Plants 1:15151 doi:10.1038/NPLANTS.2015.151
Hess N, Klode M, Anders M, Sauter M (2011) The hypoxia responsive transcription factor genes ERF71/HRE2 and ERF73/HRE1 of Arabidopsis are differentially regulated by ethylene. Physiol Plant 143:41–49
Hinz M, Wilson IW, Yang J, Buerstenbinder K, Llewellyn D, Dennis ES, Sauter M, Dolferus R (2010) Arabidopsis RAP2.2: an ethylene response transcription factor that is important for hypoxia survival. Plant Physiol 153:757–772
Hirayama T, Fujishige N, Kunii T, Nishimura N, Iuchi S, Shinozaki K (2004) A novel ethanol-hypersensitive mutant of Arabidopsis. Plant Cell Physiol 45:703–711
Hoeren FU, Dolferus R, Wu YU, Peacock WJ, Dennis ES (1998) Evidence for a role of AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase gene (adh1) by low oxygen. Genetics 149:479–490
Jiao YH, Sun LR, Song YL, Wang LM, Liu LP, Zhang LY, Liu B, Li N, Miao C, Hao FS (2013) AtrbohD and AtrbohF positively regulate abscisic acid inhibited primary root growth by affecting Ca2+ signaling and auxin response of roots in Arabidopsis. J Exp Bot 64:4183–4192
Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22:2623–2633
Li N, Sun LR, Zhang LY, Song YL, Hu PP, Li C, Hao FS (2015) AtrbohD and AtrbohF negatively regulate lateral root development by changing the localized accumulation of superoxide in primary roots of Arabidopsis. Planta 241:591–602
Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LACJ, Perata P, van Dongen JT (2011) Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479:419–422
Liu F, Vantoai T, Moy L, Bock G, Linford LD, Quackenbush J (2005) Global transcription profiling reveals novel insights into hypoxic response in Arabidopsis. Plant Physiol 137:1115–1129
Lokdarshi A, Conner WC, McClintock C, Li T, Roberts DM (2016) Arabidopsis CML38, a calcium sensor that localizes to ribonucleoprotein complexes under hypoxia stress. Plant Physiol 170:1046–1059
Ma LY, Zhang H, Sun LR, Jiao YH, Zhang GZ, Miao C, Hao FS (2012) NADPH oxidase AtrbohD and AtrbohF function in ROS dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J Exp Bot 63:305–317
Maruta T, Inoue T, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S (2011) Arabidopsis NADPH oxidases, AtrbohD and AtrbohF, are essential for jasmonic acid-induced expression of genes regulated by MYC2 transcription factor. Plant Sci 180:655–660
Morales J, Kadota Y, Zipfel C, Molina A, Torres MA (2016) The Arabidopsis NADPH oxidases RbohD and RbohF display differential expression patterns and contributions during plant immunity. J Exp Bot 67:1663–1676
Mori IC, Schroeder JI (2004) Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol 135:702–708
Mustroph A, Albrecht G (2003) Tolerance of crop plants to oxygen deficiency stress: fermentative activity and photosynthetic capacity of entire seedlings under hypoxia and anoxia. Physiol Plant 117:508–520
Papdi C, Pérez-Salamó I, Joseph MP, Giuntoli B, Bögre L, Koncz C, Szabados L (2015) The low oxygen, oxidative and osmotic stress responses synergistically act through the ethylene response factor VII genes RAP2.12, RAP2.2 and RAP2.3. Plant J 82:772–784
Paul MV, Iyer S, Amerhauser C, Lehmann M, van Dongen JT, Geigenberger P (2016) Oxygen sensing via the ethylene response transcription factor RAP2.12 affects plant metabolism and performance under both normoxia and hypoxia. Plant Physiol 172:141–153
Pucciariello C, Parlanti S, Banti V, Novi G, Perata P (2012) Reactive oxygen species-driven transcription in Arabidopsis under oxygen deprivation. Plant Physiol 159:184–196
Shabala S, Shabala L, Barcelo J, Poschenrieder C (2014) Membrane transporters mediating root signaling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ 37:2216–2233
Shahzad Z, Canut M, Tournaire-Roux C, Martinière A, Boursiac Y, Loudet O, Maurel C (2016) A potassium-dependent oxygen sensing pathway regulates plant root hydraulics. Cell 167:87–98
Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. PNAS 99:523–528
Voesenek LACJ, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytol 206:57–73
Wang FF, Chen ZH, Liu XH, Colmer TD, Zhou MX, Shabala S (2016) Tissue-specific root ion profiling reveals essential roles of the CAX and ACA calcium transport systems in response to hypoxia in Arabidopsis. J Expt Bot 67:3747–3762
Wilkins KA, Matthus E, Swarbreck SM, Davies JM (2016) Calcium-mediated abiotic stress signaling in roots. Front Plant Sci 7:1296. doi:10.3389/fpls.2016.01296
Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawaguchi K, Oyanagi A, Nakazono M (2014) Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot 65:261–273
Yang CY (2014) Hydrogen peroxide controls transcriptional responses of ERF73/HRE1 and ADH1 via modulation of ethylene signaling during hypoxic stress. Planta 239:877–885
Yang CY, Hong CP (2015) The NADPH oxidase RbohD is involved in primary hypoxia signalling and modulates expression of hypoxia-inducible genes under hypoxic stress. Environ Exp Bot 115:63–72
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30970235 and 31070239), Science and Technology Development Program of He’nan in China (162102110005), and Foundation of He’nan Educational Committee of China (15A210018, 17A180018 and 14B180029).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Chun-Hai Dong.
Rights and permissions
About this article
Cite this article
Liu, B., Sun, L., Ma, L. et al. Both AtrbohD and AtrbohF are essential for mediating responses to oxygen deficiency in Arabidopsis . Plant Cell Rep 36, 947–957 (2017). https://doi.org/10.1007/s00299-017-2128-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2128-x