Abstract
Key message
Allocation of the chromosome 2D of Ae. tauschii in triticale background resulted in changes of its organization, what is related to varied expression of genes determining agronomically important traits.
Abstract
Monosomic alien addition lines (MAALs) are crucial for transfer of genes from wild relatives into cultivated varieties. This kind of genetic stocks is used for physical mapping of specific chromosomes and analyzing alien genes expression. The main aim of our study is to improve hexaploid triticale by transferring D-genome chromatin from Aegilops tauschii × Secale cereale (2n = 4x = 28, DDRR). In this paper, we demonstrate the molecular cytogenetics analysis and SSR markers screening combined with phenotype analysis and evaluation of powdery mildew infection of triticale monosomic addition lines carrying chromosome 2D of Ae. tauschii. We confirmed the inheritance of chromosome 2D from the BC2F4 to the BC2F6 generation of triticale hybrids. Moreover, we unveiled a high variable region on the short arm of chromosome 2D, where chromosome rearrangements were mapped. These events had direct influence on plant height of hybrids what might be connected with changes at Rht8 loci. We obtained 20 semi-dwarf plants of BC2F6 generation carrying 2D chromosome with the powdery mildew resistance, without changes in spike morphology, which can be used in the triticale breeding programs.
Similar content being viewed by others
Introduction
The world production of hexaploid triticale (×Triticosecale Wittm.) is rising from 473 thousand tonnes in 2012 to 820 thousand tonnes in 2014 (FAOSTAT 2015). The worldwide expansion of triticale exposed the crop to a variety of stressful environmental conditions (Arseniuk and Góral 2015). From the other side, the global market requires diversified forms of triticale considering grain quality, resistance against biotic and abiotic stresses, and plant morphology. Considering triticale breeding, one of the main goals is to transfer genes of interest from wild relatives into cultivated varieties. The ability of triticale to be crossed with related species allows the addition of the whole genomes or individual alien chromosomes to the triticale complement. Monosomic alien addition lines (MAALs) are genotypes with an alien chromosome from a donor species added to the genome of recipient species. This kind of genetic stocks is widely used for physical mapping of specific chromosomes (Kynast et al. 2004) and analyzing alien genes expression (Cho et al. 2006). The production of alien addition lines and the introgressions with alien chromosome segments carrying target traits can be induced by homoeologous recombination or the gametocidal effect (Kwiatek et al. 2016a, b).
Ae. tauschii is a wild, diploid goatgrass (donor of D-genome to bread wheat), characterized by wide genetic variation and close related to the species of the Triticeae tribe. Because of this relationship and variety of genes, this species has been exploited by various groups around the world especially for wheat improvement (Ogbonnaya et al. 2005). The chromosome 2D of Ae. tauschii is bearing two important agronomically genes Rht8 and Pm43 as well as bh-D1 a multirow spike recessive allele (alias mrs1) determining the supernumerary spikelets trait (Jia et al. 2013). The variation of genes on one chromosome indicates that it is the most valuable one for breeding improvement of Triticeae species.
In triticale breeding programs, reduction of plant height may affect to a better partitioning of assimilates for the benefit of the spike and reducing the risk of lodging what leads to increasing of grain yield (Foulkes et al. 2011). Recently, the height of the cereal plants can be changed by breeders through the use of dwarfing or semi-dwarfing genes. The phenomenon of introduction of the reduced height (Rht) genes into bread wheat (Triticum aestivum L.) was the major event of ‘green revolution’ (Hedden 2003) and is widely used in wheat breeding. Particulary, the modest height-reducing gene Rht8 may be suitable to reduce the height of mature plants without compromising early plant growth. The genetic linkage maps presented by Korzun et al. (1998) place the wheat microsatellite marker WMS 261 (Xgwm261 in map of Somers et al. 2004) 0.6 cM distal to Rht8 on the short arm of chromosome 2D.
The most important trait for breeding is yield. One of the factors determining this agronomic feature is the amount of grain per plant, which indicates the productiveness of given genotypes. It especially depends on the architecture of the inflorescences. The number of spikelets per rachis node is a key taxonomic trait of the Triticeae tribe. The wheat and rye spike normally bears one spikelet per rachis node, and the formation of supernumerary spikelets (SS) is rare. The loci responsible for the ‘multirow spike’ or MRS trait in wheat and the ‘monstrosum spike’ trait in rye are under the control of a recessive allele at a single locus. The Mrs1 locus is located on chromosome 2DS and the Mo1 locus on chromosome 2RS. Furthermore, there is also identified a homologous loci on the chromosome 2AS of hexaploid, tetraploid, and diploid wheats.
In the last few years, the cultivation of triticale was limited due to the infection by Blumeria graminis f. sp. tritici which caused powdery mildew (Pm) (Czembor et al. 2013). Therefore, resistant varieties are the most feasible means of controlling the disease and reducing yield losses. To date, 54 Pm resistance genes where transferred from the wild relatives to wheat (Zhan et al. 2014). However, in the case of triticale, only one work reports the positive transfer of Pm13 gene from Aegilops variabilis (Kwiatek et al. 2016a). Ae. tauschii genomes constitute a great source of Pm resistance because of the occurrence of Pm2, Pm34, and Pm35 genes on chromosome 5D and Pm19 on chromosome 7D (Genesymbol, McIntosh et al. 2003). Furthermore, Jia et al. (2013) mapped Pm43 gene in Ae. tauschii chromosome 2D, in which the origin source has heretofore been Th. intermedium. So far, Th. intermedium resistance gene (later called Pm43) was recently found in partial amphiploids with wheat, in the substitution line 2 J(2D), in which a J-chromosome of Th. intermedium was substituted for chromosome 2D in wheat (Liu and Wang 2005; Liu et al. 2005) as well as transfer to wheat using a resistant partial amphiploid as a bridging parent in crosses with susceptible wheat lines.
Our main goal was to produce triticale MAALs carrying 2D chromosome of Ae. tauschii. For that reason, we have introduced D-genome chromosomes into triticale using Aegilops tauschii Coss. (DD, 2n = 2x = 14) × S. cereale (RR, 2n = 2x = 14) amphiploid forms to hybridize with triticale cv. Bogo (Kwiatek et al. 2015). The present study aimed: (1) to characterize the chromosome composition of the BC2F4 to BC2F6 generations of Ae. tauschii × triticale hybrids; (2) to verify the D-genome composition with selected SSR markers in hybrid plants, and (3) to evaluate the influence of the D-genome introgression onto important agronomic traits, including plant height, spike morphology, and resistance to powdery mildew in comparison with winter triticale cultivar Bogo.
Materials and methods
Plant material
Glasshouse experiments were carried out in three subsequent vegetation seasons at the Institute of Plant Genetics, Polish Academy of Sciences in Poznań, Poland. Seeds of Aegilops tauschii Coss. (D51; 2n = 2x = 14; DD), S. cereale (Strzękęcińskie; 2n = 2x = 14; RR) and ×Triticosecale Wittm. (Bogo; 2n = 6x = 42; AABBRR) originating from the collection of the Institute of Plant Genetics. The Ae. tauschii × S. cereale amphiploids (2n = 4x = 28; DDRR) were obtained using embryo rescue by Sulinowski and Wojciechowska of the Institute of Plant Genetics (data unpublished). The F1 (Ae. tauschii × S. cereale) × triticale hybrids were obtained by crossing of triticale cv. Bogo with Ae. tauschii × S. cereale amphiploids as a pollinator. Backcrosses with the triticale as a male parent were used to achieve following generations (BC1F1 and BC2F1) and further self-crossed to produce following generations of BC2F2 to BC2F6 hybrid plants. Seeds of T. aestivum cv. Chinese Spring were kindly supplied by the National Small Grains Collection (USDA-ARS).
Probe labeling
Total genomic DNA was extracted from fresh leaves of Ae. tauschii (DD), Triticum monococcum (AmAm), Ae. speltoides (BB), S. cereale (RR), and triticale ‘Bogo’ (AABBRR) using GeneMATRIX Plant and Funghi DNA Purification Kit (EURx Ltd.). Genomic DNA from Ae. tauschii and T. monococcum were labeled by nick translation (using NickTranslation Kit, Roche, Mannheim, Germany) with tetramethyl-5-dUTP-rhodamine (Roche) or digoxigenin-11-dUTP (Roche) depending on the visualization concept. Blocking DNA from triticale, Ae. speltoides and S. cereale, was sheared to fragments of 5–10 kb by boiling for 30–45 min and used at a ratio of 1:50 (probe:block). The 5S rDNA and 25S rDNA as well as pSc119.2 and pAs1 probes were obtained as have been described by Kwiatek et al. (2016a, b) and labeled with tetramethyl-rhodamine-5-dUTP (Roche), digoxigenin-11-dUTP (Roche), digoxigenin-11-dUTP (Roche), and tetramethyl-rhodamine-5-dUTP (Roche), respectively. Digoxigenin detection was made using anti-digoxigenin-fluorescein antibody (Roche).
Chromosome preparation and in situ hybridization
Germination, metaphase accumulation, and fixation procedures were carried out according to Kwiatek et al. (2016a). The chromosome preparations were made according to Hasterok et al. (2006). FISH and GISH experiments were performed for the identification of chromatin introgression. The analysis of genomic composition of hybrid plants was carried on the mitotic chromosomes of root meristems. Four probes (5S rDNA, 25S rDNA, pSc119.2, and pAs1) were subjected to in situ hybridization on the same chromosome preparations. First FISH was made according to Książczyk et al. (2011) with minor modifications of Kwiatek et al. (2013) using 5S rDNA (pTa794) and 25S rDNA (used for the detection of 25-5.8-18S rDNA loci). The hybridization mixture (40 μl per slide) contained 90 ng of each probe in the presence of salmon sperm DNA, 50 % formamide, 2 × SSC, and 10 % dextran sulphate, and was denatured at 75 °C for 10 min and stored on ice for 5 min. Chromosomal DNA was denatured in the presence of the hybridization mixture at 75 °C for 5 min on a heating table (Medax) and allowed to hybridize overnight at 37 °C. Digoxigenin detection was made using anti-digoxigenin-fluorescein antibody (Roche). After documentation of the FISH sites, the slides were washed according to procedure of Heslop-Harrison (2000) (2 × 45 min in 4 × SSC Tween and 2 × 5 min in 2 × SSC, at room temperature). Second FISH with pSc119.2 and pAs1 was made with the same conditions after reprobing followed by GISH carried out according to Kwiatek et al. (2012) with modifications. GISH experiments were performed using D-genome probe (from Ae. tauschii) and unlabelled triticale genomic DNA which was used as specific blocker. Multicolour GISH (mcGISH) was carried out using A-genome probe (from T. monococcum), D-genome probe (from Ae. tauschii) as well as unlabelled Ae. speltoides and S. cereale genomic DNA which were used as specific blockers. The GISH mixture (40 μL per slide), containing 50 % formamide, 2 × SSC, 10 % dextran sulphate, 90 ng each of the genome probes, and 4.5 μg blocking DNA, was denatured at 75 °C for 10 min and stored on ice for 10 min. The chromosomal DNA denaturation, hybridization, and immunodetection conditions were the same as in FISH experiments. Mitotic cells were examined with an Olympus BX 61 automatic epifluorescence microscope with Olympus XM10 CCD camera. Image processing was carried out using the Olympus Cell-F (version 3.1; Olympus Soft Imaging Solutions GmbH: Münster, Germany) imaging software and PaintShop Pro X5 software (version 15.0.0.183; Corel Corporation, Ottawa, Canada). The identification of particular chromosomes was made by comparing the signal pattern of selected probes (5S rDNA, 25S rDNA, pSc119.2, and pAs1) according to a previous study (Kwiatek et al. 2013, 2015) and similar cytogenetic analysis (Cuadrado and Jouve 1994; Schneider et al. 2003, 2005; Wiśniewska et al. 2013).
PCR amplification of SSR markers
Genomic DNA was extracted from fresh leaves of single plants using GeneMATRIX Plant and Funghi DNA Purification Kit (EURx Ltd.). Total genomic DNAs of Ae. tauschii, ×Triticosecale cv. Bogo, T. aestivum cv. Chinese Spring, BC2F5, and BC2F6 hybrids were used as templates for PCR. Analyses were performed with 25 μl mixture according to Kwiatek et al. (2016a). PCR reactions were carried out in LabCycler thermocycler (SensoQuest Biomedizinische Elektronik, Goettingen, Germany) according to programs reported in GrainGenes 2.0 Database (http://wheat.pw.usda.gov/GG2/index.shtml) for every SSR marker. Amplification products were electrophoresed at 120 V for about 2 h in 2 % agarose gel (Sigma), stained with ethidium bromide (Sigma), visualized under UV light and photographed (Syngen UV visualiser).
Analysis of plant height, spike morphology, and effectiveness of self-pollinations
Measurements of plants height were performed when plants reached maturity. There were measured the length of stem and spike separately for every stem of the plants (1–8), and the mean values were calculated. For every plant, the spike morphology was evaluated and all the spikes were archived by photography. After the harvest, grains from spikes were threshed and the mean effectiveness of sell-pollinations for every plant was calculated. The number of obtained seeds was divided by the number of flowers in spike and expressed as a percentage value. For triticale cv. Bogo, ten representative plants were subjected to analysis regarding all mentioned phenotypic features.
Evaluation of powdery mildew infections
During the vegetation period, the level of powdery mildew natural infection was evaluated according to COBORU (the Research Centre for Cultivar Testing) recommendations on a 9° scale, where 9 is the most favourable state for agriculture. The means of powdery mildew expression scores in BC2F5 and BC2F6 hybrids, Ae. tauschii, and triticale ‘Bogo’ were compared each year to the results of PCR amplification of Pm43 marker using ANOVA calculations and Tukey’s HSD test.
Results
Identification of Ae. tauschii chromatin introgression in triticale hybrids
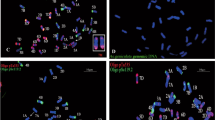
The chromosome composition of (Ae. tauschii × S. cereale) × triticale cv. Bogo hybrids was performed using FISH and GISH. The analysis were made using FISH with 5S and 25S rDNA (Fig. 1a), pSc119.2 and pAs1 (Fig. 1b), GISH with D-genome probe, and blocking DNA from triticale (Fig. 1c), as well as mcGISH with A- and D-genome probes and blocking DNA from Ae. speltoides and rye (Fig. 1d). Identification of particular chromosomes of A-, B-, R-, and D-genome was made basing on previous reports of Cuadrado and Jouve (2002), Schneider et al. (2003, 2005) and Molnár et al. (2014), respectively, and chromosome arms ratio. The analysis of F1 to BC2F3 generations was performed by Kwiatek et al. (2015) and revealed that one hybrid plant from BC2F2 exhibited 46 chromosomes with additional pairs of 2D and 3D chromosomes. This plant was self-crossed to produce BC2F3 generation. The analysis of the progeny revealed six plants with additional pairs of 2D and 3D chromosomes as well as four plants with the addition of single chromosomes 2D and four plants with single chromosome 3D. The BC2F4 to BC2F6 plants in this study were obtained only from BC2F3 genotypes characterized by constitution of 21” + 1”3D + 1”2D chromosomes by subsequent self-pollinations (Fig. 2). FISH experiments allowed to distinguish 1 plant of BC2F4 with additional pair of 2D chromosomes. Eight plants of BC2F5 generation consisted of five plants with introgression of single chromosome 2D. Another three plants carried one rearranged chromosome 2D considering the differences in pAs1 sequence signals pattern (Fig. 3). Furthermore, 36 plants of BC2F6 generation were characterized by the presence of individual chromosome 2 of D-genome chromatin. Among BC2F6 hybrid plants, twenty carried single chromosome 2D, whereas sixteen carried one rearranged chromosome 2D.
Mitotic chromosomes of BC2F5 (Ae. tauschii × S. cereale) × Triticosecale cv. Bogo hybrid analyzed using a FISH with 5S rDNA (red) and 35S rDNA (green) probes, b FISH with pAs1 (red) and pSc119.2 (green) probes, c GISH with total genomic DNA probes of Ae. tauschii (D, green) and triticale (ABR, orange), d multicolor GISH with total genomic DNA probes of T. monococcum (A, green), S. cereale and Ae. speltoides (R and B, blue), and Ae. tauschii (D, red). Arrows indicate the introgressed chromosome 2D. Scale bar 10 µm (color figure online)
SSR markers analysis specific for D-genome chromatin
The analysis of molecular markers, specific for every D-genome chromosome according to wheat (T. aestivum cv. Chinese Spring) genetic maps reported by Somers et al. (2004) (Xcfd19-1D; Xgwm301-2D; Xbarc71-3D; Xcfd71-4D; Xcfd10-5D; Xcfd49-6D; Xgwm428-7D) confirmed the FISH/GISH results. The presence of 2D chromosome in eight plants of BC2F5 generation was confirmed in comparison with positive controls, which consists of Ae. tauschii and negative control of × Triticosecale cv. Bogo (Fig. 4). Furthermore, the organization of this chromosome was analyzed using 13 SSR markers (Xcfd56-2DS; Xgdm35-2DS; Xcfd51-2DS; Xwmc25-2DS; Xwmc503-2DS; Xgwm261-2DS; Xgwm296-2DS; Xgwm210-2DS; Xgwm455-2DS; Xgwm102-2DS; Xgwm157-2DL; Xgwm539-2DL; Xgwm301-2DL) with special consideration of the short arm, where Rht8 is located. SSR markers’ analysis provided polymorphic sizes of bands considering three hybrid plants of BC2F5 generation. It was revealed that the place of rearrangement occurred between markers Xcfd51 and Xgwm210 in the short arm of 2D chromosome. The highest polymorphism was observed considering the sizes of amplification products of Xgwm261 marker which is linked to Rht8 gene (Fig. 4). The occurrence of the rearrangement in this region was also observed in plants of the next generation (BC2F6). It is worth to mention that in the case of most SSR markers, the size of the bands differs between selected Ae. tauschii line D51 and T. aestivum cv. Chinese Spring, however, appropriate bands were not present in triticale cv. Bogo. Weak bands appeared in negative control considering Xgwm539, Xgwm301, Xgdm35, and Xgwm157 markers, which were detected in hybrid plants, as well.
Molecular analysis of selected SSRs specific for chromosome 2D in genomes of hybrid plants with a rearranged chromosome 2D; a Xgwm261, b Xgwm301, c Xgwm539, d Xgwm210; hybrid plants with rearranged 2D chromosome (1), hybrid plants with whole 2D chromosome (2), Ae. tauschii (3), T. aestivum cv. Chinese Spring (4) and triticale cv. Bogo (5). Marker (M) size 100 bp
Evaluation of the influence of 2D chromosome addition on plant height and spike morphology
In general, all monosomic alien addition plants of BC2F5 generation were lower in comparison with triticale (Table 1). The mean height of hybrid plants was 68 cm, whereas triticale plants were 26 cm higher (94 cm) what indicates about 28 % of height reduction. All hybrid plants of BC2F5 were treated as one group due to the small number of plants with D-genome introgression. In turn, all BC2F6 hybrids (36 plants) were divided into two groups: plants with chromosome 2D and plants which rearranged chromosome 2D according to the results of cytogenetic and SSR markers analysis. This observation revealed that plants with chromosome 2D and rearranged chromosome 2D lead to the decrease of plant height of about 37 (39 %) and 12 cm (12.5 %), respectively, in comparison with triticale (96 cm). When all hybrid plants of BC2F6 were treated as one group, such as in previous season, the mean value and reduction of plant heights were similar in both years (data not shown). The spike morphology was normal among BC2F5 hybrid plants in comparison with triticale cv. Bogo. In contrast, spikes of 16 hybrid plants of the next generation (22 % of all hybrids of BC2F6 generation) were characterized by the presence of supernumerary spikelets (SS) generally in the lower third of spike. However, there are also three plants which can be characterized by many additional spikelets along the whole length of the spikes (Fig. 5a). This trait in BC2F6 generation of hybrids leads to the increased fertility (64 %) in comparison with hybrid plants demonstrating spikes similar to normal triticale (46 %) (Fig. 5b), whereas triticale cv. Bogo (Fig. 5c) revealed high fertility (90 %) (Table 2). Comparing the mean fertility values for both generations of hybrids, the obtained results were similar and indicate about 44 % reduction of grains yield in comparison with triticale. Analysis with Xgwm102 marker localized nearby mrs1 in bread wheat revealed that the amplification product of 150 bp size was present in all analyzed hybrid plants and Ae. tauschii and was not present in triticale cv. Bogo.
Evaluation of expression of the resistance to powdery mildew provided by Pm43 gene from Ae. tauschii in triticale monosomic addition plants carrying 2D chromosome
During the vegetation period, all analyzed plants of Ae. tauschii exhibited low level of powdery mildew natural infection and mean scores ranged between 8.3 and 8.45 (Table 3). The observations of the infection symptoms conducted on all analyzed monosomic addition plants of BC2F5 and BC2F6 generations showed high tolerance to powdery mildew. There were no differences in resistance between hybrids carrying normal 2D and rearranged 2D chromosome. The mean scores of infection ranged between 7.63 and 7.97. In comparison, triticale cv. Bogo plants were less tolerant and the mean scores of the infection level ranged between 2.85 and 3.05 (Table 3). This observation was in accordance with the results of the SSR markers analysis with Xgwm539 marker, located on chromosome 2D of Ae. tauschii, which is one of the markers related to powdery mildew resistant gene Pm43 (Fig. 4). The amplification products of 150 bp in size were found in DNA extracts of Ae. tauschii (positive control carrying Pm43) and all monosomic addition plants of BC2F5 and BC2F6 generations. There were no differences in resistance between hybrids carrying normal 2D and rearranged 2D chromosome. The marker for Pm43 was not identified in triticale ‘Bogo’ (negative control).
Discussion
The two groups of chromosomes within Triticeae tribe are considered to posses high density of genes (Conley et al. 2004). According to the literature, chromosome 2D of Aegilops tauschii especially carries the Rht8 gene which determine semi-dwarfism feature and Pm43 gene related to the resistance to Blumeria graminis (Jia et al. 2013). Furthermore, this chromosome bears gene mrs1 responsible for SS (supernumerary spikelets) trait in bread wheat (Dobrovolskaya et al. 2015). In this work, we investigate the influence of the 2D chromosome of Ae. tauschii in monosomic addition plants of triticale considering agronomically important traits.
One of our assumptions was to introduce Rht8 gene determining semi-dwarfism in wheat to triticale using Ae. tauschii monosomic addition lines, which will be employed for 2R/2D substitution or translocation induction. In our work, we used Ae. tauschii × S. cereale amphiploids as a bridge forms for the introgression of D-genome chromatin to triticale. Such a comprehensive approach was applied by Kwiatek et al. (2016a), who transfer the Pm13 gene from Ae. variabilis × S. cereale amphiploids into triticale cv. Lamberto. The present results show that introgression of 2D chromosomes, bearing Rht8 gene, from Ae. tauschii into triticale chromosome complement was successful and led to the height reduction of monosomic alien addition plants. Furthermore, this feature was maintained in the subsequent generations of hybrid plants. All monosomic alien addition plants, namely, 8 of BC2F5 and 36 plants of BC2F6, for which PCR amplification revealed bands for Xgwm261 marker, were substantially lower than triticale. The presence of the Ae. tauschii type band (200 bp) in hybrids can be associated with the reduction of height of about 37 cm in comparison with triticale cv. Bogo which mean height was 96 cm. Similarly, 16 hybrids in which rearrangement event took place were also lower than triticale; however, the mean reduction of height was 12 cm only (Table 1). The presence of Rht8 in wheat leads up to 10 cm reduction of the European varieties (Worland et al. 1998); however, T. aestivum is lower than triticale generally because of the presence of the remaining semi-dwarfism genes. The absence of these genes with the presence of the “alien” semi-dwarfism gene Rht8 in winter triticale is most likely the general reason of such high decreasing of plants height. Furthermore, our analysis revealed differences in size of amplification products of Xgwm261 marker between positive controls—Ae. tauschii and T. aestivum cv. Chinese Spring. The amplification products of 200 bp in size were characteristic to Ae. tauschii and monosomic alien addition plants of BC2F5 and BC2F6 generations. SSR analysis of hybrid plants carrying rearranged 2D chromosome revealed amplification products of about 140 bp. Band of predicted size 192 bp was obtained for DNA of positive control ‘Chinese Spring’. The marker for Rht8 was not identified in triticale cv. Bogo. That observations indicate that Ae. tauschii posses different alleles of Rht8 gene, where wheat ‘Chinese Spring’ can be characterized by the most frequent allele Rht8c. According to McIntosh et al. (2003) analyzed Ae. tauschii accession might posses allele Rht8d (201 bp) because of the similarity of amplification product size.
Monosomic addition lines of triticale carrying chromosome 2D were also investigated in terms of powdery mildew resistance identification. According to Jia et al. (2013), a powdery mildew resistance gene Pm43 was mapped on the 2D chromosome of wheat. The molecular marker analysis and the visual evaluation of powdery mildew symptoms in Ae. tauschii revealed the presence of Pm43 marker (Xgwm539) and low powdery mildew reaction, confirmed by infection scores made on 20 plants each year of the experiment (Table 3). Similarly, monosomic alien addition plants of the BC2F5 and BC2F6 generations were highly tolerant to powdery mildew infection and possessed Pm43 marker. A lack of the differences in resistance between hybrids with additional 2D and rearranged 2D chromosome is related to the localization of the Pm43 marker on the long arm of chromosome 2D. In comparison, triticale ‘Bogo’ was much more infected, which was confirmed by Tukey’s HSD test (Table 3). It is in accordance with the results of Czembor et al. (2013) who reported that triticale ‘Bogo’ is susceptible to all isolates of Blumeria graminis derived from triticale. Furthermore, the molecular analysis showed that Pm43 marker was not present in triticale ‘Bogo’ (Fig. 4; Table 3). Pm43 gene was mapped on the basis of CH5025 × CH5065 2DL population source map (Jia et al. 2013). CH5025 was a Th. intermedium-derived line of wheat resistant to powdery mildew, whereas CH5065 is susceptible line (He et al. 2009). On this basis, it can be assumed that the region determining resistance to powdery mildew in Th. intermedium is homologous in Ae. tauschii what is consistent with observations of He et al. (2009) about chromosome pairing of D-genome of wheat and J or Js chromosomes of Th. intermedium. Furthermore, in both the species as well as in obtained hybrids, the resistance was maintained at both the stages of development. The successful transfer of 2D chromosomes and positive effect of powdery mildew resistance in triticale proved that Ae. tauschii is more valuable source of this gene for breeding programs because of the simplest genome composition and closer relationship with traditional crops, rather than Th. intermedium.
Our work revealed a rearrangement of chromosome considering additional chromosome 2D in the subsequent generations of triticale plants. The chromosome aberrations appeared between markers Xcfd51 and Xgwm210 in the short arm of chromosome 2D. This region is notably important forasmuch, and there is localized semi-dwarfing gene Rht8. The changes in organization of 2D chromosome had direct influence on plant height of triticale hybrids, what might be connected with changes in Rht8 loci which is localized nearby Xgwm261. Although the occurrence of rearrangement was unexpected in such advance generations of plants, its localization in chromosome is not random and might be explained by the evolution of Ae. tauschii genome. According to the literature, based on the sequencing data, it was hypothesized that the seven chromosomes of this species originated from 12 ancestral chromosomes by five nested chromosome insertions (NCIs). During the NCIs, a telomere of the inserted chromosome was inserted near the centromere in a gene-containing region. As a result of NCIs, one of the centromere was lost, and the centromere of the inserted chromosome became the active centromere in each compound chromosome. Chromosome 2D of Ae. tauschii was produced as a result of the NCIs. Therefore, it might be concluded that examined in this research region is specially exposed to chromosome rearrangements and such places in genome may constitute a hot spots. Furthermore, the heat map of the recombination rates showed that the recombination rate for this region is relatively high (Luo et al. 2009, 2013). That might be the reason of rearrangement events which occur especially during stress conditions, such as integration of the alien chromatin into hybrid plants or backcrossing.
The results of this research showed that the presence of additional chromosomes 2D induces changes in spike morphology of triticale hybrid plants. However, measurements of spikes revealed any significant differences in the length of the spike in hybrid plants (data not shown). Most of the analyzed plants were characterized by the presence of spikes which morphology was congenial to triticale spike what should be expected in advanced generations of hybrids (Fig. 5b). However, about 22 % of BC2F6 generation plants posses spikes with supernumerary spikelets, a trait which was already reported only for wheat, where the MRS trait is under the control of a recessive allele at a single locus located on chromosome 2D (Dobrovolskaya et al. 2015). The presence of bh-D1, a multirow spike recessive allele (alias mrs1), was also reported for Ae. tauschii (Jia et al. 2013). SSR marker analysis with Xgwm102 marker revealed amplification products of 150 bp in size for Ae. tauschii and unexpectedly for all hybrid plants. Considering the obtained results and wheat origin of this SSR marker (genetic distance 1.3 cM), it was assumed that this marker is not suitable to analyze the SS trait in hybrid plants of Ae. tauschii and triticale. This relevance proves that the genetic distance between bh-D1 and Xgwm102 marker on map reported by Jia et al. (2013) is 9.43 cM which indicates that this markers in not combined with SS trait in Ae. tauschii. It is also important that this recessive trait in triticale hybrids was conditioned only by the presence of single chromosome 2D which is consistent with the observations of Sears (1954), who report the reduplication of spikelets in hexaploid wheat plants nullisomic for this chromosomes. The occurrence of SS trait in analyzed plants leads to the increase of grain yield in comparison with the rest pool of plants (Table 2). These observations allowed to conclude that such a approach may allow new spike architectures of triticale to be designed, with the aim of enhancing grain production.
In conclusion, monosomic alien addition plants obtained here provide a feasible platform to identify and estimate valuable traits for triticale breeding using molecular cytogenetics, screening of forms with SSR markers combined with phenotype analysis, and evaluation of powdery mildew infection. Using these methods, we have obtained 20 semi-dwarf plants of BC2F6 generation carrying 2D chromosome with the powdery mildew resistance, without changes in spike morphology which can be used in the triticale breeding programs. Furthermore, the limitations of reports about transferring the powdery mildew resistance genes as well as genes determining semi-dwarfism and spike morphology to triticale indicate that there is a great legitimacy and potential in the field of using monosomic addition plants carrying chromosomes of Ae. tauschii to improve this valuable crop. From the other hand, the molecular analysis of triticale monosomic alien addition plants provided significant insights considering the organization changes of additional chromosome induced by the integration of the alien chromatin in triticale genetic background.
References
Arseniuk E, Góral T (2015) Chapter 5. Triticale biotic stresses–known and novel foes, In: Eudes F (ed.), Triticale, Springer International Publishing, Berlin, p 83–108
Cho S, Garvin DF, Muehlbauer GJ (2006) Transcriptome analysis and physical mapping of barley genes in wheat–barley chromosome addition lines. Genetics 172:1277–1285
Conley et al (2004) A 2600-locus chromosome bin map of wheat homoeologous group 2 reveals interstitial gene-rich islands and colinearity with rice. Genetics 168:625–637
Cuadrado A, Jouve N (1994) Mapping and organization of highly repeated DNA sequences by means of simultaneous and sequential FISH and C-banding in 6x-triticale. Chromosome Res 2:331–338
Cuadrado A, Jouve N (2002) Evolutionary trends of different repetitive dna sequences during speciation in the genus Secale. J Hered 93:339–345
Czembor HJ, Doraczyńska O, Czembor JH (2013) In Polish: Odporność odmian pszenżyta na mączniaka prawdziwego (Blumeria graminis ff. ssp.) występującego w Polsce (Resistance of triticale cultivars to powdery mildew (Blumeria graminis ff. ssp.) occurring in Poland). Biuletyn Instytutu Hodowli i Aklimatyzacji Roślin 267:3–16
Dobrovolskaya O et al (2015) FRIZZY PANICLE drives supernumerary spikelets in bread wheat. Plant Physiol 167:189–199
FAOSTAT (2015) Production Crops. (http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor). Accessed 20 Nov 2015
Foulkes MJ, Slafer GA, Davies WJ, Berry PM, Sylvester-Bradley R, Martre P, Calderini DF, Griffiths S, Reynolds MP (2011) Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J Exp Bot 62:469–486
Hasterok R, Dulawa J, Jenkins G, Leggett M, Langdon T (2006) Multisubstrate chromosome preparations for high throughput comparative FISH. BMC Biotechnol 6:1–5
He R, Chang Z, Yang Z, Yuan Z, Zhan H, Zhang X, Liu J (2009) Inheritance and mapping of powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor Appl Genet 118:1173–1180
Hedden P (2003) The genes of the Green Revolution. Trends in Genetics 19:5–9
Heslop-Harrison JS (2000) Comparative genome organization in plants: from sequence and markers to chromatin and chromosomes. Plant Cell 12:617–635
Jia et al (2013) Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 496:91–95
Korzun V, Roder MS, Ganal MW, Worland AJ, Law CN (1998) Genetic analysis of the dwarfing gene (Rht8) in wheat. Part I. Molecular mapping of Rht8 on the short arm of chromosome 2D of bread wheat (Triticum aestivum L.). Theor Appl Genet 96:1104–1109
Książczyk T, Apolinarska B, Kulak-Książczyk S, Wiśniewska H, Stojałowski S, Łapiński M (2011) Identification of the chromosome complement and the spontaneous 1R/1 V translocations in allotetraploid Secale cereale × Dasypyrum villosum hybrids through cytogenetic approaches. J Appl Genet 52:305–311
Kwiatek M, Błaszczyk L, Wiśniewska H, Apolinarska B (2012) Aegilops-Secale amphiploids: chromosome categorisation, pollen viability and identification of fungal disease resistance genes. J Appl Genet 53:37–40
Kwiatek M, Wiśniewska H, Apolinarska B (2013) Cytogenetic analysis of Aegilops chromosomes, potentially usable in triticale (×Triticosecale Witt.) breeding. J Appl Genet 54:147–155
Kwiatek M, Majka M, Wiśniewska H, Apolinarska B, Belter J (2015) Effective transfer of chromosomes carrying leaf resistance genes from Aegilops tauschii Coss. into hexaploid triticale (X Triticosecale Witt.) using Ae. tauschii × Secale cereale amphiploid form. J Appl Genet 56:163–168
Kwiatek M, Belter J, Majka M, Wiśniewska H (2016a) Allocation of the S-genome chromosomes of Aegilops variabilis Eig. carrying powdery mildew resistance in triticale (× Triticosecale Wittmack). Protoplasma 253:329–343
Kwiatek M, Majka M, Ślusarkiewicz-Jarzina A, Ponitka A, Pudelska H, Belter J, Wiśniewska H (2016b) Inheritance of the Aegilops ovata chromosomes carrying gametocidal factors in hexaploid triticale (×Triticosecale Wittm.) hybrids. J Appl Genet. doi:10.1007/s13353-015-0332-3
Kynast RG, Okagaki RJ, Galatowitsch MW, Granath SR, Jacobs MS, Stec AO, Rines HW, Phillips RL (2004) Dissecting the maize genome by using chromosome addition and radiation hybrid lines. Proc Natl Acad Sci USA 101:9921–9926
Liu SB, Wang HG (2005) Characterization of a wheat-Thinopyron intermedium substitution line with resistance to powdery mildew. Euphytica 143:229–233
Liu SB, Wang HG, Zhang XY, Li XF, Li DY, Duan XY, Zhou YL (2005) Molecular Cytogenetic identification of a wheat-Thinopyron intermedium (Host) Barkworth and DR Dewey partial amphiploid resistant to powdery mildew. J Integr Plant Biol 47:726–733
Luo MC et al (2009) Genome comparisons reveal a dominant mechanism of chromosome number reduction in grasses and accelerated genome evolution in Triticeae. PNAS 106:15780–15785
Luo MC et al (2013) A 4-gigabase physical map unlocks the structure and evolution of the complex genome of Aegilops tauschii, the wheat D-genome progenitor. PNAS 110:7940–7945
McIntosh RA, Yamazaki Y, Devos KM, Dubcovsky J, Rogers WJ et al. (2003) Catalogue of gene symbols for wheat. In: Pogna NE, Romano M, Pogna E, Galterio G (eds.) Proceedings of the 10th International Wheat Genetics Symposium, Vol. 4, Instituto Sperimentale per la Cerealicotura, Rome, p 1–34
Molnár I, Kubaláková M, Šimková H, Farkas A, Cseh A, Megyeri M, Vrána J, Molnár-Láng M, Doležel J (2014) Flow cytometric chromosome sorting from diploid progenitors of bread wheat, T. urartu, Ae. speltoides and Ae. tauschii. Theor Appl Genet 127:1091–1104
Ogbonnaya FC, Halloran GM, Lagudah ES (2005) D genome of wheat–60 years on from Kihara, Sears and McFadden. In: Tsunewaki K (ed) Frontiers of Wheat Bioscience. Kihara memorial foundation for the advancement of life sciences, Yokohama
Schneider A, Linc G, Molnár-Láng M (2003) Fluorescence in situ hybridization polymorphism using two repetitive DNA clones in different cultivars of wheat. Plant Breed 122:396–400
Schneider A, Linc G, Molnár I, Molnár-Lang M (2005) Molecular cytogenetic characterization of Aegilops biuncialis and its use for the identification of 5 derived wheat–Aegilops biuncialis addition lines. Genome 48:1070–1082
Sears ER (1954) The aneuploids of common wheat. University of Missouri, Columbia, pp 3–58
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114
Wiśniewska H, Kwiatek M, Kulak-Książczyk S, Apolinarska B (2013) Introgression of A- and B-genome chromatin into tetraploid rye (Secale cereale L.). J Appl Genet 54:435–440
Worland AJ, Korzun V, Roder MS, Ganal MW, Law CN (1998) Genetic analysis of the dwarfing gene Rht8 in wheat. Part II. The distribution and adaptive significance of allelic variants at the Rht8 locus of wheat as revealed by microsatellite screening. Theor Appl Genet 96:1110–1120
Zhan HX, Li GR, Zhang XJ, LI X, Guo HJ, Gong WP, Jia JQ, Qiao LY (2014) Chromosomal location and comparative genomics analysis of powdery mildew resistance gene Pm51 in a putative wheat-Thinopyrum ponticum introgression line. PLoS One 9:e113455
Acknowledgments
We would like to gratefully acknowledge the technical assistance of Mrs. Grażyna Cicha and Mrs. Joanna Maszner. This work was financed by the National Science Centre (DEC-2014/15/N/NZ9/00448).
Author contribution statement
MM., M.K., and H.W. initiated the project and designed the study. M.K., M.M., and J.B. performed the research. M.M. wrote the paper. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by I. Hwang.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Majka, M., Kwiatek, M., Belter, J. et al. Characterization of morphology and resistance to Blumeria graminis of winter triticale monosomic addition lines with chromosome 2D of Aegilops tauschii . Plant Cell Rep 35, 2125–2135 (2016). https://doi.org/10.1007/s00299-016-2023-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-2023-x