Abstract
Key message
Overexpression of OsGS gene modulates oxidative stress response in rice after exposure to cadmium stress. Our results describe the features of transformants with enhanced tolerance to Cd and abiotic stresses.
Abstract
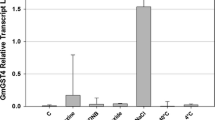
Glutamine synthetase (GS) (EC 6.3.1.2) is an enzyme that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine. Exposure of plants to cadmium (Cd) has been reported to decrease GS activity in maize, pea, bean, and rice. To better understand the function of the GS gene under Cd stress in rice, we constructed a recombinant pART vector carrying the GS gene under the control of the CaMV 35S promoter and OCS terminator and transformed using Agrobacterium tumefaciens. We then investigated GS overexpressing rice lines at the physiological and molecular levels under Cd toxicity and abiotic stress conditions. We observed a decrease in GS enzyme activity and mRNA expression among transgenic and wild-type plants subjected to Cd stress. The decrease, however, was significantly lower in the wild type than in the transgenic plants. This was further validated by the high GS mRNA expression and enzyme activity in most of the transgenic lines. Moreover, after 10 days of exposure to Cd stress, increase in the glutamine reductase activity and low or no malondialdehyde contents were observed. These results showed that overexpression of the GS gene in rice modulated the expression of enzymes responsible for membrane peroxidation that may result in plant death.








Similar content being viewed by others
References
Abdula SE, Lee HJ, Melgar RJ, Sun MM, Kang KK, Kim DS, Cho YG (2011) Isolation, characterization and overexpression of Bradh1 gene from Brassica rapa encoding alcohol dehydrogenase increases germinating rice seeds under anaerobic condition. J Plant Biotechnol 38:77–86
Astolfi S, Zuchi S, Passera C (2004) Role of sulphur availability on cadmium-induced changes of nitrogen and sulphur metabolism in maize (Zea mays L.) leaves. J Plant Physiol 161:795–802
Balestrasse KB, Gallego SM, Tomaro ML (2006) Oxidation of the enzymes involved in nitrogen assimilation plays an important role in the cadmium-induced toxicity in soybean plants. Plant Soil 284:187–194
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Boussama N, Ouariti O, Ghorbal MH (1999) Changes in growth and nitrogen assimilation in barley seedlings under cadmium stress. J Plant Nutr 22:731–752
Bradford MM (1976) A dye binding assay for protein. Anal Biochem 72:248–254
Brugière N, Dubois F, Masclaux C, Sangwan RS, Hirel B (2000) Immunolocalization of glutamine synthetase in senescing tobacco (Nicotiana tabacum L.) leaves suggests that ammonia assimilation is progressively shifted to the mesophyll cytosol. Planta 211:519–527
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cai H, Zhou Y, Xiao J, Li X, Zhang Q, Lian X (2009) Overexpressed glutamine synthetase gene modifies nitrogen metabolism and abiotic stress responses in rice. Plant Cell Rep 28:527–537
Chaoui A, Mazhoudi S, Ghorbal MH, El Ferjani E (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Chien HF, Kao CH (2000) Accumulation of ammonium in rice leaves in response to excess cadmium. Plant Sci 156:111–115
Chien HF, Lin CC, Wang JW, Chen CT, Kao CH (2002) Changes in ammonium ion content and glutamine synthetase activity in rice leaves caused by excess cadmium area consequence of oxidative damage. Plant Growth Regul 36:41–47
Cho YG, Kang HJ, Lee JS, Lee YT, Lim SJ, Gauch H, Eun MY, McCouch SR (2007) Identification of quantitative trait loci in rice for yield, yield components, and agronomic traits across years and locations. Crop Sci 47:2403–2417
Chugh LK, Gupta VK, Sawhney SK (1992) Effect of cadmium on enzymes of nitrogen metabolism in pea seedlings. Phytochemistry 31:395–400
di Toppi S, Gabbrieli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Edwards JW, Walker E, Coruzzi GM (1990) Cell specific expression in transgenic plants reveals non-overlapping roles for chloroplast and cytosolic glutamine synthetase. Proc Natl Acad Sci USA 87:3459–3463
Elstner EF (1991) Mechanisms of oxygen activation in different compartments of plant cells. In: Pell EJ, Steffen KL (eds) Active oxygen species, oxidative stress, and plant metabolism. American Society of Plant Physiologists, Rockville, pp 13–25
Gallego SM, Benavides MP, Tomaro ML (1996) Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci 121:151–159
Gebhardt C, Oliver JE, Forde BG, Saarelainen R, Miflin BJ (1986) Primary structure and differential expression of glutamine synthetase genes in nodules, roots and leaves of Phaseolus vulgaris. EMBO J 5:1429–1435
Gleave AP (1992) A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mole Biology 20:1203–1207
Gouia H, Ghorbel MH, Meyer C (2000) Effects of cadmium on activity of nitrate reductase and on other enzymes of nitrate assimilation pathway in bean. Plant Physiol Biochem 38:629–638
Hare P, Cress W (1997) Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regul 21:79–102
Hassan MJ, Shao G, Zhang G (2005) Influence of cadmium toxicity on growth and antioxidant enzyme activity in rice cultivars with different cadmium accumulation. J Plant Nutr 28:1259–1270
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. 1. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem and Biophys 125:189–198
Hoshida H, Tanaka Y, Hibino T, Hayashi Y, Tanaka A, Takabe T, Takabe T (2000) Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol Biol 43:103–111
Hsu YT, Kao CH (2004) Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul 42:227–238
Husic DW, Husic HD, Tolbert NE (1987) The oxidative photosynthetic carbon cycle or C2 cycle. CRC Crit Rev Plant Sci 5:45–100
Husted S, Mattsson M, Mollers C, Wallbraun M, Schjoerring JK (2002) Photorespiratory NH4 + production in leaves of wild-type and glutamine synthetase 2 antisense oilseed rape. Plant Physiol 130:989–998
Jana S, Choudhuri M (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354
Lee HJ, Abdula SE, Jee MG, Jang DW, Cho YG (2011) High-efficiency and Rapid Agrobacterium-mediated genetic transformation method using germinating rice seeds. J Plant Biotechnol 38:251–257
Lee HJ, Abdula SE, Cho YG (2012) Overexpression of OsMLD Encoding MYB-like DNA binding domain increases tolerance to salt stress in rice (Oryza sativa L.) Kor J Breed Sci 44:100–109
Lozano-Rodriguez E, Hernandez LE, Bonzy P, Charpena-Ruiz RO (1997) Distribution of cadmium in shoot and root tissue of maize and pea plants: physiological disturbances. J Exp Bot 306:123–128
Miflin BJ, Lea PJ (1976) The pathway of nitrogen assimilation in plants. Phytochemistry 15:873–885
Mittova V, Theodoulou FL, Kiddle G, Gomez L, Volokita M, Tal M (2003) Co-ordinate induction of glutathione biosynthesis and glutathione metabolizing enzymes is correlated with salt tolerance. FEBS Lett 554:417–442
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610
O’Neal D, Joy KW (1973) Glutamine synthetase of pea leaves: purification, stabilization and pH optima. Arch Biochem Biophys 159:113–122
Ogren WL (1984) Photorespiration: pathways, regulation and modification. Annu Rev Plant Physiol 35:415–442
Oliveira IC, Brears T, Knight TJ, Clark A, Coruzzi GM (2002) Overexpression of cytosolic glutamine synthetase: relation to nitrogen, light and photorespiration. Plant Physiol 129:1170–1180
Ouariti O, Gouia H, Ghorbal MH (1997) Responses of bean and tomato plants to cadmium: growth, mineral nutrition and nitrate reduction. Plant Physiol Biochem 35:347–354
Pandolfini T, Gabbrielli R, Comparini C (1992) Nickel toxicity and peroxidase activity in seedlings of Triticum aestivum L. Plant Cell Environ 15:719–725
Ranieri A, Castanga A, Scebba F, Careri M, Zagnoni I, Predieri G, Pagliari M, Sanita di Topi L (2005) Oxidative stress and phytochelatin characterisation in bread wheat exposed to cadmium excess. Plant Physiol Biochem 43:45–54
Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-B- and ozone induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136
Sekmen AH, Turkan I, Takio S (2007) Differential responses to antioxidant enzymes and lipid peroxidation of salt stress in salt tolerant Plantago maritime and salt-sensitive Plantago media. Physiol Plant 131:399–411
Smith IK, Vierheller TL, Thorne CA (1988) RAssay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal Biochem 175:408–413
Somashekaraiah BV, Padmaja K, Prasad ARK (1992) Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): involvement of lipid peroxides in chlorophyll degradation. Physiol Plant 85:85–89
Stobart A, Griths W, Ameen-Bukhari I, Sherwood P (1985) The effect of Cd2+ on the biosynthesis of chlorophyll in leaves of barley. Physiol Plantarum 63:293–298
Sun MM, Abdula SE, Lee HY, Cho YC, Han LZ, Koh HJ, Cho YG (2011) Molecular aspect of good eating quality formation in Japonica rice. PLoS ONE 6(4):e18385. doi:10.1371/journal.pone.0018385
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Tobin AK, Yamaya T (2001) Cellular compartmentation of ammonium assimilation in rice and barley. J Exp Bot 356:591–604
Troll W, Lindsley J (1955) A photometric method for the determination of proline. J Biochem 215:655–660
Verma K, Shekhawat GS, Sharma A, Mehta SK, Sharma V (2008) Cadmium induced oxidative stress and changes in soluble and ionically bound cell wall peroxidase activities in roots of seedling and 3–4 leaf stage plants of Brassica juncea (L.) czern. Plant Cell Rep 27:1261–1269
Wagner GJ (1993) Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron 51:173–213
Wallsgrove RM, Lea PJ, Miflin BJ (1979) Distribution of enzymes of nitrogen assimilation within the pea leaf cell. Plant Physiol 63:232–236
Wojcik M, Vangronsveld J, Tukiendorf A (2005) Cadmium tolerance in Thlaspi caerulescens. I. Growth parameters, metal accumulation and phytochelatins synthesis in response to cadmium. Environ Exp Bot 53:151–161
Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259:181–189
Yannarelli GG, Fernández-Alvarez AJ, Santa-Cruz DM, Tomaro ML (2007) Glutathione reductase activity and isoforms in leaves and roots of wheat plants subjected to cadmium stress. Phytochemistry 68:505–512
Zhang FQ, Shi WY, Jin ZX, Shen ZG (2003) Response of antioxidative enzymes in cucumber chloroplasts to cadmium toxicity. J Plant Nutr 26:1779–1788
Acknowledgments
This study was supported by a grant from the Next-Generation BioGreen 21 Program (Project No. PJ009008), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. S. Shin.
Rights and permissions
About this article
Cite this article
Lee, H.J., Abdula, S.E., Jang, D.W. et al. Overexpression of the glutamine synthetase gene modulates oxidative stress response in rice after exposure to cadmium stress. Plant Cell Rep 32, 1521–1529 (2013). https://doi.org/10.1007/s00299-013-1464-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1464-8