Abstract
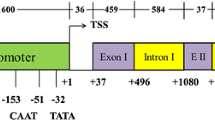
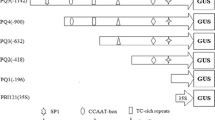
Ubiquitin is an abundant protein involved in protein degradation and cell cycle control in plants and rubi3 is a polyubiquitin gene isolated from rice (Oryza sativa L.). Using both GFP and GUS as reporter genes, we analyzed the expression pattern of the rubi3 promoter as well as the effects of the rubi3 5′-UTR (5′ untranslated region) intron and the 5′ terminal 27 bp of the rubi3 coding sequence on the activity of the promoter in transgenic rice plants. The rubi3 promoter with the 5′-UTR intron was active in all the tissue and cell types examined and supported more constitutive expression of reporter genes than the maize Ubi-1 promoter. The rubi3 5′-UTR intron mediated enhancement on the activity of its promoter in a tissue-specific manner but did not alter its overall expression pattern. The enhancement was particularly intense in roots, pollen grains, inner tissue of ovaries, and embryos and aleurone layers in maturing seeds. The translational fusion of the first 27 bp of the rubi3 coding sequence to GUS gene further enhanced GUS expression directed by the rubi3 promoter in all the tissues examined. The rubi3 promoter should be an important addition to the arsenal of strong and constitutive promoters for monocot transformation and biotechnology.







Similar content being viewed by others
Abbreviations
- 5′-UTR:
-
5′ Untranslated region
- CaMV:
-
Cauliflower mosaic virus
- DIC:
-
Differential interference contrast
- GFP:
-
Green fluorescent protein
- GUS:
-
β-Glucuronidase
- IME:
-
Intron-mediated enhancement of gene expression
- MU:
-
4-Methylumbelliferone
- MUG:
-
4-Methylumbelliferyl-β-d-glucoside
- NOS:
-
Nopaline synthase
References
Atkinson HJ, Grimwood S, Johnston K, Green J (2004) Prototype demonstration of transgenic resistance to the nematode Radopholus similis conferred on banana by a cystatin. Transgenic Res 13:135–142
Bachmair A, Becker F, Masterson RV, Schell J (1990) Perturbation of the ubiquitin system causes leaf curling, vascular tissue alterations and necrotic lesions in a higher-plant. EMBO J 9:4543–4549
Bhattacharjee B, An G, Gupta HS (1997) Fertile transgenic Indica rice produced by expression of maize ubiquitin promoter-bar chimaeric gene in the protoplasts. J Plant Biochem Biotechnol 6:69–73
Binet MN, Lepetit M, Weil JH, Tessier LH (1991) Analysis of a sunflower polyubiquitin promoter by transient expression. Plant Sci 79:87–94
Butaye KMJ, Cammue BPA, Delaure SL, De Bolle MFC (2005) Approaches to minimize variation of transgene expression in plants. Mol Breed 16:79–91
Chaubet-Gigot N, Kapros T, Flenet M, Kahn K, Gigot C, Waterborg JH (2001) Tissue-dependent enhancement of transgene expression by introns of replacement histone H3 genes of Arabidopsis. Plant Mol Biol 45:17–30
Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes—structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18:675–689
Cornejo MJ, Luth D, Blankenship KM, Anderson OD, Blechl AE (1993) Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol 23:567–581
Fu HY, Kim SY, Park WD (1995a) A potato Sus3 sucrose synthase gene contains a context-dependent 3′ element and a leader intron with both positive and negative tissue-specific effects. Plant Cell 7:1395–1403
Fu HY, Kim SY, Park WD (1995b) High-level tuber expression and sucrose inducibility of a potato Sus4 sucrose synthase gene require 5′-flanking and 3′-flanking sequences and the leader intron. Plant Cell 7:1387–1394
Gallomeagher M, Irvine JE (1993) Effects of tissue-type and promoter strength on transient GUS expression in sugarcane following particle bombardment. Plant Cell Rep 12:666–670
Gallomeagher M, Irvine JE (1996) Herbicide resistant transgenic sugarcane plants containing the bar gene. Crop Sci 36:1367–1374
Garbarino JE, Oosumi T, Belknap WR (1995) Isolation of a polyubiquitin promoter and its expression in transgenic potato plants. Plant Physiol 109:1371–1378
Genschik P, Marbach J, Uze M, Feuerman M, Plesse B, Fleck J (1994) Structure and promoter activity of a stress and developmentally-regulated polyubiquitin-encoding gene of Nicotiana tabacum. Gene 148:195–202
Hansen G, Chilton MD (1999) Lessons in gene transfer to plants by a gifted microbe. Plant Biotechnol 240:21–57
Hill-Ambroz KL, Weeks JT, Baenziger PS, Graybosch RA (2001) Constitutive promoter expression of transgenes in wheat (Triticum aestivum). Cereal Res Commun 29:9–16
Hobbs SLA, Warkentin TD, Delong CMO (1993) Transgene copy number can be positively or negatively associated with transgene expression. Plant Mol Biol 21:17–26
Hochstrasser M (1996) Ubiquitin-dependent protein degradation. Annu Rev Genet 30:405–439
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene-transfer to plants. Transgenic Res 2:208–218
Ingham DJ, Beer S, Money S, Hansen G (2001) Quantitative real-time PCR assay for determining transgene copy number in transformed plants. Biotechniques 31:132
Jin SG, Komari T, Gordon MP, Nester EW (1987) Genes responsible for the supervirulence phenotype of Agrobacterium tumefaciens A281. J Bacteriol 169:4417–4425
Jorgensen RA, Cluster PD, English J, Que QD, Napoli CA (1996) Chalcone synthase cosuppression phenotypes in petunia flowers: Comparison of sense vs antisense constructs and single-copy vs complex T-DNA sequences. Plant Mol Biol 31:957–973
Kamo K, Blowers A, McElroy D (2000) Effect of the cauliflower mosaic virus 35S, actin, and ubiquitin promoters on uidA expression from a bar-uidA-fusion gene in transgenic Gladiolus plants. In Vitro Cell Dev Biol Plant 36:13–20
King RW, Deshaies RJ, Peters JM, Kirschner MW (1996) How proteolysis drives the cell cycle. Science 274:1652–1659
Lu J, Sivamani E, Azhakanandam K, Samadder P, Li X, Qu R (2008) Gene expression enhancement mediated by the 5′ UTR intron of the rice rubi3 gene varied remarkably among tissues in transgenic rice plants. Mol Genet Genomics 279:563–572
Mascarenhas JP, Hamilton DA (1992) Artifacts in the localization of GUS activity in anthers of petunia transformed with a CaMV 35S-GUS Construct. Plant J 2:405–408
Muhitch MJ, Shatters RG (1998) Regulation of the maize ubiquitin (Ubi-1) promoter in developing maize (Zea mays L.) seeds examined using transient gene expression in kernels grown in vitro. Plant Cell Rep 17:476–481
Norris SR, Meyer SE, Callis J (1993) The intron of Arabidopsis thaliana polyubiquitin genes is conserved in location and is a quantitative determinant of chimeric gene expression. Plant Mol Biol 21:895–906
Plesse B, Criqui MC, Durr A, Parmentier Y, Fleck J, Genschik P (2001) Effects of the polyubiquitin gene Ubi.U4 leader intron and first ubiquitin monomer on reporter gene expression in Nicotiana tabacum. Plant Mol Biol 45:655–667
Rechsteiner M (1991) Natural substrates of the ubiquitin proteolytic pathway. Cell 66:615–618
Rollfinke IK, Silber MV, Pfitzner UM (1998) Characterization and expression of a heptaubiquitin gene from tomato. Gene 211:267–276
Rooke L, Byrne D, Salgueiro S (2000) Marker gene expression driven by the maize ubiquitin promoter in transgenic wheat. Ann Appl Biol 136:167–172
Samadder P, Sivamani E, Lu J, Li X, Qu R (2008) Transcriptional and post-transcriptional enhancement of gene expression by the 5′ UTR intron of rice rubi3 gene in transgenic rice cells. Mol Genet Genomics 279:429–439
Schledzewski K, Mendel RR (1994) Quantitative transient gene-expression—comparison of the promoters for maize polyubiquitin1, rice actin1, maize-derived Emu and CaMV 35S in cells of barley, maize and tobacco. Transgenic Res 3:249–255
Sheen J, Hwang SB, Niwa Y, Kobayashi H, Galbraith DW (1995) Green-fluorescent protein as a new vital marker in plant-cells. Plant J 8:777–784
Sit TL, Vaewhongs AA, Lommel SA (1998) RNA-mediated trans-activation of transcription from a viral RNA. Science 281:829–832
Sivamani E, Qu R (2006) Expression enhancement of a rice polyubiquitin gene promoter. Plant Mol Biol 60:225–239
Stoger E, Williams S, Keen D, Christou P (1999) Constitutive versus seed specific expression in transgenic wheat: temporal and spatial control. Transgenic Res 8:73–82
Streatfield SJ, Magallanes-Lundback ME, Beifuss KK, Brooks CA, Harkey RL, Love RT, Bray J, Howard JA, Jilka JM, Hood EE (2004) Analysis of the maize polyubiquitin-1 promoter heat shock elements and generation of promoter variants with modified expression characteristics. Transgenic Res 13:299–312
Takimoto I, Christensen AH, Quail PH, Uchimiya H, Toki S (1994) Non-systemic expression of a stress-responsive maize polyubiquitin gene (Ubi-1) in transgenic rice plants. Plant Mol Biol 26:1007–1012
Toki S, Takamatsu S, Nojiri C, Ooba S, Anzai H, Iwata M, Christensen AH, Quail PH, Uchimiya H (1992) Expression of a maize ubiquitin gene promoter-bar chimeric gene in transgenic rice plants. Plant Physiol 100:1503–1507
Wang J, Oard JH (2003) Rice ubiquitin promoters: deletion analysis and potential usefulness in plant transformation systems. Plant Cell Rep 22:129–134
Wang JL, Jiang JD, Oard JH (2000) Structure, expression and promoter activity of two polyubiquitin genes from rice (Oryza sativa L.). Plant Sci 156:201–211
Wang MB, Waterhouse PM (2000) High-efficiency silencing of a beta-glucuronidase gene in rice is correlated with repetitive transgene structure but is independent of DNA methylation. Plant Mol Biol 43:67–82
Weeks JT, Anderson OD, Blechl AE (1993) Rapid production of multiple independent lines of fertile transgenic wheat (Triticum aestivum). Plant Physiol 102:1077–1084
Wei H, Albert HH, Moore PH (1999) Differential expression of sugarcane polyubiquitin genes and isolation of promoters from two highly-expressed members of the gene family. J Plant Physiol 155:513–519
Wei HR, Wang ML, Moore PH, Albert HH (2003) Comparative expression analysis of two sugarcane polyubiquitin promoters and flanking sequences in transgenic plants. J Plant Physiol 160:1241–1251
Wilmink A, Vandeven BCE, Dons JJM (1995) Activity of constitutive promoters in various species from the Liliaceae. Plant Mol Biol 28:949–955
Woffenden BJ, Freeman TB, Beers EP (1998) Proteasome inhibitors prevent tracheary element differentiation in Zinnia mesophyll cell cultures. Plant Physiol 118:419–429
Acknowledgments
We thank Drs. N. S. Allen, E. Johannes, and J. Xu for their technical assistance with the imaging work at CMIF, NCSU, the Plant Analysis Group at Syngenta Biotechnology, Inc., for assaying the transgene copy number using realtime PCR, Dr. K. Azhakanandam for technical assistance in rice transformation, as well as Drs. J. Thomas, J. Shurtleff, and C. Saravitz, and J. Edwards for assistance in using the North Carolina State University Phytotron facility for plant maintenance. We also wish to thank Dr. T. Sit for providing the plasmid pRTL2-sGFP and Dr. P. Samadder for the plasmid pPSRG30. We are grateful to Drs. G. Allen, N. S. Allen, R. Dewey, and J. Nicholson for helpful discussions on the project. This work was supported in part by grants from the Center for Turfgrass Environmental Research and Education, North Carolina State University, The Consortium for Plant Biotechnology Research, Inc., and Syngenta Biotechnology, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Ozias-Akins.
Rights and permissions
About this article
Cite this article
Lu, J., Sivamani, E., Li, X. et al. Activity of the 5′ regulatory regions of the rice polyubiquitin rubi3 gene in transgenic rice plants as analyzed by both GUS and GFP reporter genes. Plant Cell Rep 27, 1587–1600 (2008). https://doi.org/10.1007/s00299-008-0577-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-008-0577-y