Abstract
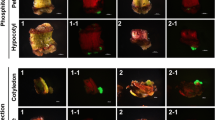
Chemical-based selection for plant transformation is associated with a number of real and perceived problems that might be avoided through visual selection. We have used green fluorescent protein (GFP), as a visual selectable marker to produce transformed papaya (Carica papaya) plants following microprojectile bombardment of embryogenic callus. GFP selection reduced the selection time from 3 months on a geneticin (G418) antibiotic-containing medium to 3–4 weeks. Moreover, GFP selection increased the number of transformed papaya plants by five-to eightfold compared to selection in the presence of antibiotics. Overall, the use of GFP for selecting transgenic papaya lines improved our throughput for transformation by 15- to 24-fold while avoiding the drawbacks associated with the use of antibiotic resistance-based selection markers.





Similar content being viewed by others
Abbreviations
- BA::
-
Benzyladenine
- 2, 4-D::
-
2,4-Dichlorophenoxyacetic acid
- GFP::
-
Green fluorescent protein
- IBA::
-
Indole-3-butyric acid
- NAA::
-
α-Naphthaleneacetic acid
- MS::
-
Murashige and Skoog plant culture medium
References
Ahlandsberg S, Satish P, Sun C, Jansson C (1999) Green fluorescent protein as a reporter system in the transformation of barley cultivars. Physiol Plant 107:194–200
Baulcombe DC, Chapman S, Santa Cruz S (1995) Jellyfish green fluorescent protein as a reporter for virus infections. Plant J 7:1045–1053
Bevan MW, Flavell RB, Chilton MD (1983) A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 304:184–187
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chalfie M, Tu Y, Euskirchen G, Ward WW, Parsher PC (1994) Green fluorescent protein as a marker for gene expression. Science 263:802–805
Cheng YH, Yang JS, Yeh SD (1996) Efficient transformation of papaya by coat protein gene of papaya ringspot virus mediated by Agrobacterium following liquid-phase wounding of embryogenic tissues with carborundum. Plant Cell Rep 16:127–132
Chiu W, Niwa Y, Zheng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 3:325–330
Comai L, Facciotti D, Hiatt WR, Thompson G, Rose RE, Stalker DM (1985) Expression in plants of a mutant aroA gene from Salmonella typhimurium confers tolerance to glyphosate. Nature 317:741–744
Crawley MJ, Brown SL, Hails RS, Kohn DD, Rees M (2001) Transgenic crops in natural habitats. Nature 409:682–683
De Block M, Botterman J, Vanderwiele M, Dockx J, Thoen C, Gossele V, Rao Movva N, Thompson C, Van Montagu M, Leemans J (1987) Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J 6:2513–2518
Ebinuma H, Sugita K, Matsunaga E, Endo S, Yamada K, Komanine A (2001) Systems for the removal of a selection marker and their combination with a positive marker. Plant Cell Rep 20:383–392
Elliott AR, Campbell JA, Dugdale B, Brettell RIS, Grof CPL (1999) Green-fluorescent protein facilitates rapid in vivo detection of genetically transformed plant cells. Plant Cell Rep 18:707–714
Fitch MMM (1993) High frequency somatic embryogenesis and plant regeneration from papaya hypocotyl callus. Plant Cell Tissue Organ Cult 32:205–212
Fitch MMM, Manshardt RM, Gonsalves D, Slightom JL, Sanford JC (1990) Stable transformation of papaya via microprojectile bombardment. Plant Cell Rep 9:189–194
Fitch MMM, Manshardt RM, Gonsalves D, Slightom JL (1993) Transgenic papaya plants from Agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep 12:245–249
Fitch MMM, Pang S-Z, Slightom JL, Lius S, Tennant P, Manshardt RM, Gonsalves D (1994) Genetic transformation in Carica papaya L. (Papaya). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 29. Springer, Berlin Heidelberg New York, pp 236–256
Fraley RT, Rogers SG, Horsch RB, Sanders PR, Flick JS, Adams SP, Bittner ML, Brand LA, Fink CL, Fry JS, Galluppi GR, Goldberg SB, Hoffmann NL, Woo SC (1983) Expression of bacterial genes in plant cells. Proc Natl Acad Sci USA 80:4803–4807
Harper BK, Mabon SA, Leffel SM, Halfhill MD, Richards HA, Moyer KA, Stewart CN Jr (1999) Green fluorescent protein as a marker for expression of a second gene in transgenic plants. Nat Biotechnol 17:1125–1129
Haseloff J, Amos B (1995) GFP in plants. Trends Genet 11:328–329
Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94:2122–2127
Haughn GW, Smith J, Mazur B, Somerville C (1988) Transformation with a mutant Arabidopsis acetolactate synthase gene renders tobacco resistant to sulfonylurea herbicides. Mol Gen Genet 211:266–271
Heim R, Prasher DC, Tsien RY (1994) Wavelength mutations and posttranslational auto-oxidation of green fluorescent protein. Proc Natl Acad Sci USA 91:12501–12504
Herrera-Estrella L, De Block M, Messens E, Hernalsteens JP, Van Montagu M, Schell J (1993) Chimeric genes as dominant selectable markers in plant cells EMBO J 2:987–995
Jefferson R (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Joersbo M, Donaldson I, Kreiber J, Petersen SG, Brunstedt J, Okkels FT (1998) Analysis of mannose selection used for transformation of sugar beet. Mol Breed 4:111–117
Kaeppler, HF, Menon GK, Skadsen RW, Nuutila AM, Carlson AR (2000) Transgenic oat plants via visual selection of cells expressing green fluorescent protein. Plant Cell Rep 19:661–666
Kaeppler HF, Carlson AR, Menon GK (2001) Routine utilization of green fluorescent protein as a visual selectable marker for cereal transformation. In Vitro Cell Dev Biol-Plant 37:120–126
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ow DW, Wood KV, de Luca M, deWet JR, Helinski DR, Howell SH (1986) Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science 234:856–859
Plautz JD, Day RN, Dailey GM, Welsh SB, Hall JC, Halpain S, Kay SA (1996) Green fluorescent protein and its derivates as versatile maker for gene expression in living Drosophila melanogaster, plant, and mammalian cells. Gene 173:83–87
Richards HA, Han CT, Hopkins RG, Failla ML, Ward WW, Stewart CN Jr (2003) Safety assessment of recombinant green fluorescent protein orally administered to weaned rats. Am Soc Nutr Sci J Nutr 133:1909–1912
Rogers SO, Bendlich AL (1994) Extraction of total cellular DNA from plants, algae and fungi. In: Gelvin SB, Schilperoort AR (eds) Plant molecular biology manual, 2nd edn. Kluwer, Dordrecht, pp 1–8
Sacchetti A, Alberti S (1999) Protein tags enhance GFP folding in eukaryotic cells. Nat Biotechnol 17:1046
Sacchetti A, Ciccocioppo R, Alberti S (2000) The molecular determinants of the efficiency of green fluorescent protein mutants. Histopathology 15:101–107
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn, vol 1–3. Cold Spring Harbor Press, Cold Spring Harbor
Siemering KR, Golbik R, Sever R, Haseloff J (1996) Mutations that suppress the thermosensitivity of green fluorescent protein. Curr Biol 6:1653–1663
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Bol 98:503–517
Stewart CN Jr (2001) The utility of green fluorescent protein in transgenic plants. Plant Cell Rep 20:376–382
Todd R, Tague BW (2001) Phosphomannose isomerase: a versatile selectable marker for Arabidopsis thaliana germ-line transformation. Plant Mol Biol Rep 19:307–319
Vain P, Worland B, Kohli A, Snape J, Christou P (2000) The green fluorescent protein (GFP) as a vital screenable marker in rice transformation. Theor Appl Genet 96:164–169
Van Den Elzen PJM, Townsend J, Lee KY, Bedbrook JR (1985) A chimaeric hygromycin resistance gene as a selectable marker in plant cells. Plant Mol Biol 5:299–302
Wright M, Dawson J, Dunder E, Suttie J, Reed J, Dramer C, Chang Y, Novitzky R, Wang H, Artim-Moore L (2001) Efficient biolistic transformation of maize (Zea mays L.) and wheat (Triticum aestivum L.) using the phosphomannose isomerase gene, pmi, as the selectable marker. Plant Cell Rep 20:429–436
Acknowledgements
This research was supported in part by a US Department of Agriculture ARS cooperative agreement No. CA58-5320-3-460 with the Hawaii Agriculture Research Center. The authors would like to thank Dr. Mingli Wang, HARC, for providing the pML202 construct, CAMBIA, Canberra, Australia, for providing the pCAMBIA1303 construct, and Dr. Henrik Albert, USDA, ARS, for thoughtful discussions and for technical assistance with the fluorescence microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.J. Rose
Rights and permissions
About this article
Cite this article
Zhu, Y.J., Agbayani, R. & Moore, P.H. Green fluorescent protein as a visual selection marker for papaya (Carica papaya L.) transformation. Plant Cell Rep 22, 660–667 (2004). https://doi.org/10.1007/s00299-004-0755-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0755-5