Abstract
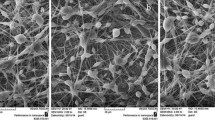
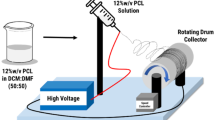
Poly(l-lactic acid) (PLLA) fiber mats containing two types of crude Garcinia mangostana Linn. (GM) extract [i.e., dichloromethane extract (dGM) and acetone extract (aGM)] were successfully prepared by electrospinning process. Both the neat and the GM-loaded PLLA fibers were smooth, with the average diameters ranging between 0.77 and 1.14 μm. The release characteristics of GM from the GM-loaded PLLA fiber mats were carried out by total immersion method in acetate buffer or simulated body fluid that contained 0.5 % v/v Tween 80 and 3 % v/v methanol (hereafter, A/T/M or S/T/M medium) at either 32 or 37 °C, respectively. The maximum cumulative amounts of GM released from the GM-loaded PLLA fiber mats in the S/T/M medium were greater than those in the A/T/M medium. Moreover, the cumulative amounts of GM released from the aGM-loaded PLLA fiber mats were greater than those from the dGM-loaded PLLA fiber mats in both types of medium. The antibacterial activity of the dGM-loaded PLLA fiber mats was greatest against Staphylococcus aureus DMST 20654, while that of the aGM-loaded PLLA fiber mats was greatest against S. aureus ATCC 25923 and S. epidermidis. Lastly, only the dGM-loaded PLLA fiber mats at extraction ratio of 10 mg mL−1 were toxic to the human dermal fibroblasts.




Similar content being viewed by others
References
Hartgerink JD, Beniash E, Stupp SI (2001) Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 294:1684–1688. doi:10.1126/science.1063187
Zhang S (2003) Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol 21:1171–1178. doi:10.1038/nbt874
Hargerink JD, Beniash E, Stupp SI (2002) Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci USA 99:5133–5138. doi:10.1073/pnas.072699999
Zong X, Ran S, Kim K-S, Fang D, Hsiao BS, Chu B (2003) Structure and morphology changes during in vitro degradation of electrospun poly(glycolide-co-lactide) nanofiber membran. Biomacromolecules 4:416–423. doi:10.1021/bm025717o
Yoshimoto H, Shin YM, Terai H, Vacanti JP (2003) A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 24:2077–2082. doi:10.1016/S0142-9612(02)00635-X
van de Witte P, Dijkstra PJ, van den Berg JWA, Feijen J (1996) Phase separation processes in polymer solutions in relation to membrane formation. J Membr Sci 117:1–31. doi:10.1016/0376-7388(96)00088-9
Fu X, Matsuyama H, Teramoto M, Nagai H (2006) Preparation of polymer blend hollow fiber membrane via thermally induced phase separation. Sep Purif Technol 52:363–371. doi:10.1016/j.seppur.2006.05.018
Reneker DH, Chun I (1996) Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 7:216–223. doi:10.1088/0957-4484/7/3/009
Qi Z, Yu H, Chen Y, Zhu M (2009) Highly porous fibers prepared by electrospinning a ternary system of nonsolvent/solvent/poly(l-lactic acid). Mater Lett 63:415–418. doi:10.1016/j.matlet.2008.10.059
Shalumon KT, Binulal NS, Selvamurugan N, Nair SV, Menon D, Furuike T, Tamura H, Jayakumar R (2009) Electrospinning of carboxymethyl chitin/poly(vinyl alcohol) nanofibrous scaffolds for tissue engineering applications. Carbohydr Polym 77:863–869. doi:10.1016/j.carbpol.2009.03.009
Gautam S, Dinda AK, Mishra NC (2013) Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater Sci Eng C Mater Biol Appl 33:1228–1235. doi:10.1016/j.msec.2012.12.015
Zhou J, Cao C, Ma X, Lin J (2010) Electrospinning of silk fibroin and collagen for vascular tissue engineering. Int J Biol Macromol 47:514–519. doi:10.1016/j.ijbiomac.2010.07.010
Kenawy E-R, Abdel-Hay FI, El-Newehy MH, Wnek GE (2009) Processing of polymer nanofibers through electrospinning as drug delivery systems. Mater Chem Phys 113:296–302. doi:10.1016/j.matchemphys.2008.07.081
Nguyen TTT, Ghosh C, Hwang S-G, Chanunpanich N, Park JS (2012) Porous core/sheath composite nanofibers fabricated by coaxial electrospinning as a potential mat for drug release system. Int J Pharm 439:296–306. doi:10.1016/j.ijpharm.2012.09.019
Moreno I, González-González V, Romero-García J (2011) Control release of lactate dehydrogenase encapsulated in poly(vinly alcohol) nanofibers via electrospinning. Eur Polym J 47:1264–1272. doi:10.1016/j.eurpolymj.2011.03.005
Suwantong O, Opanasopit R, Ruktanonchai U, Supaphol P (2007) Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance. Polymer 48:7546–7557. doi:10.1016/j.polymer.2007.11.019
Montazer M, Malekzadeh SB (2012) Electrospun antibacterial nylon nanofibers through in situ synthesis of nanosilver: preparation and characteristics. J Polym Res 19:9980. doi:10.1007/s10965-012-9980-8
Suwantong O, Pankongadisak P, Deachathai S, Supaphol P (2012) Electrospun poly(l-lactic acid) fiber mats containing a crude Garcinia cowa extract for wound dressing applications. J Polym Res 19:9896. doi:10.1007/s10965-012-9896-3
Gu S-Y, Wang Z-M, Ren J, Zhang C-Y (2009) Electrospinning of gelatin and gelatin/poly(L-lactide) blend and its characteristics for wound dressing. Mat Sci Eng C 29:1822–1828. doi:10.1016/j.msec.2009.02.010
Zhou Y, Yang H, Liu X, Mao J, Gu S, Xu W (2013) Electrospinning of carboxyethyl chitosan/poly(vinyl alcohol)/silk fibroin nanoparticles for wound dressings. Int J Biol Macromol 53:88–92. doi:10.1016/j.ijbiomac.2012.11.013
Doshi J, Reneker DH (1995) Electrospinning process and applications of electrospun fibers. J Electrostat 35:151–160. doi:10.1016/0304-3886(95)00041-8
Deitzel JM, Kleinmeyer JD, Hirvonen JK, Beck Tan NC (2001) Controlled deposition of electrospun poly(ethylene oxide) fibers. Polymer 42:8163–8170. doi:10.1016/S0032-3861(01)00336-6
Long L-X, Yuan X-B, Chang J, Zhang Z-H, Gu M-Q, Song T-T, Xing Y, Yuan X-Y, Jiang S-C, Sheng J (2012) Self-assembly of polylactic acid and cholesterol-modified dextran into hollow nanocapsules. Carbohydr Polym 87:2630–2637. doi:10.1016/j.carbpol.2011.11.032
Li F, Li X, Li B (2011) Preparation of magnetic polylactic acid microspheres and investigation of its releasing property for loading curcumin. J Magn Magn Mater 323:2770–2775. doi:10.1016/j.jmmm.2011.05.045
Giurea A, Klein TJ, Chen AC, Goomer RS, Coutts RD, Akeson WH, Amiel D, Sah RL (2003) Adhesion of perichondiral cells to a polylactic acid scaffold. J Orthop Res 21:584–589. doi:10.1016/S0736-0266(02)00263-2
Hsu S-H, Chan S-H, Chiang C-M, Chen CC-C, Jiang C-F (2011) Peripheral nerve regeneration using a microporous polylactic acid asymmetric conduit in a rabbit long-gap sciatic nerve transection model. Biomaterials 32:3764–3775. doi:10.1016/j.biomaterials.2011.01.065
Zeng J, Xu X, Chen X, Liang Q, Bian X, Yang L, Jing X (2003) Biodegradable electrospun fibers for drug delivery. J Control Release 92:227–231. doi:10.1016/S0168-3659(03)00372-9
Thakur RA, Florek CA, Kohn J, Michniak BB (2008) Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int J Pharm 364:87–93. doi:0.1016/j.ijpharm.2008.07.033
Chuysinuan P, Chimnoi N, Techasakul S, Supaphol P (2009) Gallic acid-loaded electrospun poly(l-lactic acid) fiber mats and their release characteristic. Macromol Chem Phys 210:814–822. doi:10.1002/macp.200800614
Suwantong O, Pankongadisak P, Deachathai S, Supaphol P (2013) The potential of electrospun poly(l-lactic acid) fiber mats containing a crude Garcinia Dulcis extract for use as wound dresssings. Chiang Mai J Sci 40:1–17
Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD (2006) Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem 54:2077–2082. doi:10.1021/jf052649z
Mahabusarakam W, Wiriyachitra P, Taylor WC (1987) Chemical constituents of Garcinia mangostana. J Nat Prod 50:474–478. doi:10.1021/np50051a021
Jinsart W, Ternai B, Buddhasukh D, Polya GM (1992) Inhibition of wheat embryo calcium-dependent protein kinase and other kinases by mangostin and gamma-mangostin. Phytochemistry 31:3711–3713. doi:10.1016/S0031-9422(00)97514-9
Chairungsrilerd N, Furukawa K, Ohta T, Nozoe S, Ohizumi Y (1996) Pharmacological properties of alpha-mangostin, a novel histamine H1 receptor antagonist. Eur J Pharmacol 4:351–356. doi:10.1016/S0014-2999(96)00562-6
Chairungsrilerd N, Furukawa K-I, Tadano T, Kisara K, Ohizumi Y (1998) Effect of γ-mangostin through the inhibition of 5-hydroxytryptamine2A receptors in 5-fluoro-α-methyltryptamine-induced head-twitch response of mice. Br J Pharmacol 123:855–862. doi:10.1038/sj.bjp.0701695
Chen L-G, Yang L-L, Wang C-C (2008) Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol 46:688–693. doi:10.1016/j.fct.2007.09.096
Nakatani K, Atsumi M, Arakawa T, Oosawa K, Shimura S, Nakahata N, Ohizumi Y (2002) Inhibition of histamine release and prostaglandin E2 synthesis by mangosteen, a Thai medicine plant. Biol Pharm Bull 25:1137–1141. doi:10.1248/bpb.25.1137
Suksamrarn S, Suwannapoch N, Phakhodee W, Thanuhiranlert J, Ratananukul P, Chimnoi N, Suksamrarn A (2003) Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem Pharm Bull 51:857–859. doi:10.1248/cpb.51.857
Nakatani K, Nakahata N, Arakawa T, Yasuda H, Ohizumi Y (2002) Inhibition of cyclooxygenase and prostaglandin E2 synthesis by gamma-mangostin, a xanthone derivative in mangosteen, in C6 rat glioma cells. Biochem Pharmacol 63:73–79. doi:10.1016/S0006-2952(01)00810-3
Mahabusarakam W, Proudfoot J, Taylor W, Croft K (2000) Inhibition of lipoprotein oxidation by prenylated xanthones derived from mangostin. Free Radical Res 33:643–659. doi:10.1080/10715760000301161
Iinuma M, Tosa H, Tanaka T, Asai F, Kobayashi Y, Shimano R, Miyauchi K (1996) Antibacterial activity of xanthones from Guttiferaeous plants against methicillin-resistant Staphylococcus aureus. J Pharm Pharmacol 48:861–865. doi:10.1111/j.2042-7158.1996.tb03988.x
Sakagami Y, Iinuma M, Piyasena KG, Dharmaratne HR (2005) Antibacterial activity of alpha-mangostin against vancomycin resistant Enterococci (VRE) and synergism with antibiotics. Phytomedicine 12:203–208. doi:10.1016/j.phymed.2003.09.012
Yu L, Zhao M, Yang B, Zhao Q, Jiang Y (2007) Phenolics from hull of Garcinia mangostana fruit and their antioxidant activities. Food Chem 104:176–181. doi:10.1016/j.foodchem.2006.11.018
Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N (2004) Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacol 90:161–166. doi:10.1016/j.jep.2003.09.048
Weecharangsan W, Opanasopit P, Sukma M, Ngawhirunpat T, Sotanaphun U, Siripong P (2006) Antioxidative and neuroprotective activities of extracts from the fruit hull of mangosteen (Garcinia mangostana Linn.). Med Princ Pract 15:281–287. doi:10.1159/000092991
Matsumoto K, Akao Y, Kobayashi E, Ohguchi K, Ito T, Tanaka T, Iinuma M, Nozawa Y (2003) Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J Nat Prod 66:1124–1127. doi:10.1021/np020546u
Yu L, Zhao M, Yang B, Bai W (2009) Immunomodulatory and anticancer activities of phenolic from Garcinia mangostana fruit pericarp. Food Chem 116:969–973. doi:10.1016/j.foodchem.2009.03.064
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med 26:1231–1237. doi:10.1016/S0891-5849(98)00315-3
Ritger PL, Peppas NA (1987) A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release 5:23–36. doi:10.1016/0168-3659(87)90034-4
Peppas NA, Khare AR (1993) Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv Drug Deliv Rev 11:1–35. doi:10.1016/0169-409X(93)90025-Y
Verreck G, Chun I, Rosenblatt J, Peeters J, Dijck AV, Mensch J, Noppe M, Brewster ME (2003) Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J Control Release 92:349–360. doi:10.1016/S0168-3659(03)00342-0
Zarena AS, Sankar KU (2009) A study of antioxidant properties from Garcinia mangostana L. pericarp extract. Acta Sci Pol Technol Aliment 8:23–34
Marinova EM, Yanishlieva NV (1997) Antioxidative activity of extracts from selected species of the family Lamiaceae in sunflower oil. Food Chem 58:245–248. doi:10.1016/S0308-8146(96)00223-3
Acknowledgments
This work was supported by the Thailand Research Fund (Grant Number: MRG5380120). We are grateful to Mae Fah Luang University for partial financial support and laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suwantong, O., Pankongadisak, P., Deachathai, S. et al. Electrospun poly(l-lactic acid) fiber mats containing crude Garcinia mangostana extracts for use as wound dressings. Polym. Bull. 71, 925–949 (2014). https://doi.org/10.1007/s00289-014-1102-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1102-9