Abstract
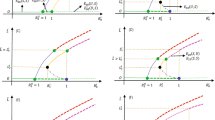
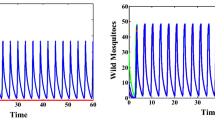
Despite centuries of continuous efforts, mosquito-borne diseases (MBDs) remain enormous health threat of human life worldwide. Lately, the USA government has approved an innovative technology of releasing Wolbachia-infected male mosquitoes to suppress the wild mosquito population. In this paper we first introduce a stage-structured model for natural mosquitos, then we establish a new model considering the releasing of Wolbachia-infected male mosquitoes and the mating competition between the natural male mosquitoes and infected males on the suppression of natural mosquitoes. Dynamical analysis of the two models, including the existence and local stability of the equilibria and bifurcation analysis, reveals the existence of a forward bifurcation or a backward bifurcation with multiple attractors. Moreover, globally dynamical properties are further explored by using Lyapunov function and theory of monotone operators, respectively. Our findings suggest that infected male augmentation itself cannot always guarantee the success of population eradication, but leads to three possible levels of population suppression, so we define the corresponding suppression rate and estimate the minimum release ratio for population eradication. Furthermore, we study how the release ratio of infected males and natural ones, mating competition, the rate of cytoplasmic incompatibility and the basic offspring number affect the suppression rate of natural mosquitoes. Our results show that the successful eradication relies on assessing the reproductive capacity of natural mosquitoes, a selection of suitable Wolbachia strains and an appropriate release amount of infected males. This study will be helpful for public health authorities in designing proper strategies to control vector mosquitoes and prevent the epidemics of MBDs.






Similar content being viewed by others
References
Abdelrazec A, Lenhart S, Zhu H (2014) Transmission dynamics of west nile virus in mosquitoes and corvids and non-corvids. J Math Biol 68(6):1553–1582
Agnew P, Haussy C, Michalakis Y (2000) Effects of density and larval competition on selected life history traits of culex pipiens quinquefasciatus (diptera: Culicidae). J Med Entomol 37(5):732–735
Anguelov R, Dumont Y, Lubuma J (2012) Mathematical modeling of sterile insect technology for control of anopheles mosquito. Comput Math Appl 64(3):374–389
Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X, Yao X, Dimopoulos G, Xi Z (2013) Wolbachia invades anopheles stephensi populations and induces refractoriness to plasmodium infection. Science 340(6133):748–751
Brelsfoard CL, Séchan Y, Dobson SL (2008) Interspecific hybridization yields strategy for south pacific filariasis vector elimination. PLoS Neglect Trop D 2(1):e129
Cai L, Ai S, Li J (2014) Dynamics of mosquitoes populations with different strategies for releasing sterile mosquitoes. SIAM J Appl Math 74(6):1786–1809
Caraballo H, King K (2014) Emergency department management of mosquito-borne illness: malaria, dengue, and west nile virus. Emerg Med Pract 16(5):1–23
Caspari E, Watson GS (1959) On the evolutionary importance of cytoplasmic sterility in mosquitoes. Evolution 13(4):568–570
Chan MHT, Kim PS (2013) Modelling a wolbachia invasion using a slow–fast dispersal reaction–diffusion approach. B Math Biol 75:1501–1523
Dean JL, Dobson SL (2004) Characterization of wolbachia infections and interspecific crosses of aedes (stegomyia) polynesiensis and ae.(stegomyia) riversi (diptera: Culicidae). J Med Entomol 41(5):894–900
Dobson SL, Fox CW, Jiggins FM (2002) The effect of wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc R Soc Lond B Bio 269(1490):437–445
Dufourd C, Dumont Y (2012) Modeling and simulations of mosquito dispersal. the case of aedes albopictus. Biomath 1(2):1209262
Dutra HLC, Rocha MN, Dias FBS, Mansur SB, Caragata EP, Moreira LA (2016) Wolbachia blocks currently circulating zika virus isolates in brazilian aedes aegypti mosquitoes. Cell Host Microbe 19(6):771–774
Farkas JZ, Hinow P (2010) Structured and unstructured continuous models for wolbachia infections. B Math Biol 72:2067–2088
Hancock PA, Sinkins SP, Charles H, Godfray J (2011) Strategies for introducing wolbachia to reduce transmission of mosquito-borne diseases. PLoS Neglect Trop D 5:e1024
Hancock PA, White VL, Ritchie SA, Hoffmann AA, Charles H, Godfray J (2016) Predicting wolbachia invasion dynamics in aedes aegypti populations using models of density-dependent demographic traits. BMC Biol 14:96
He S, Zhang X, Liang J, Tang S (2017) Multiscale modelling the effects of CI genetic evolution in mosquito population on the control of dengue fever. Sci Rep UK 7(1):13895
Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45:371–391
Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y et al (2011) Successful establishment of wolbachia in aedes populations to suppress dengue transmission. Nature 476(7361):454–457
Hu L, Huang M, Tang M, Yu J, Zheng B (2015) Wolbachia spread dynamics in stochastic environments. Theor Popul Biol 106:32–44
Huang MG, Tang MX, Yu JS (2015) Wolbachia infection dynamics by reaction–diffusion equations. Sci China Math 58(1):77–96
Hussain M, Lu G, Torres S, Edmonds JH, Kay BH, Khromykh AA, Asgari S (2013) Effect of wolbachia on replication of west nile virus in a mosquito cell line and adult mosquitoes. J Virol 87(2):851–858
Jansen VAA, Turelli M, Godfray HCJ (2008) Stochastic spread of wolbachia. Proc R Soc Lond B Biol 275:2769–2776
Kambris Z, Cook PE, Phuc HK, Sinkins SP (2009) Immune activation by life-shortening wolbachia and reduced filarial competence in mosquitoes. Science 326(5949):134–136
Keeling MJ, Jiggins FM, Read JM (2003) The invasion and coexistence of competing wolbachia strains. Heredity 91:382
Korobeinikov A, Wake GC (2002) Lyapunov functions and global stability for sir, sirs, and sis epidemiological models. Appl Math Lett 15(8):955–960
Lambrechts L, Ferguson NM, Harris E, Holmes EC, McGraw EA, O’Neill SL, Ooi EE, Ritchie SA, Ryan PA, Scott TW et al (2017) Assessing the epidemiological effect of wolbachia for dengue control. Lancet Infect Dis 15:862–866
Laven H (1967) Eradication of culex pipiens fatigans through cytoplasmic incompatibility. Nature 216(5113):383–384
Li MY, Graef JR, Wang L, Karsai J (1999) Global dynamics of a seir model with varying total population size. Math Biosci 160(2):191–213
Li Y, Liu X (2017) An impulsive model for wolbachia infection control of mosquito-borne diseases with general birth and death rate functions. Nonlinear Anal RWA 37:412–432
Li Y, Liu X (2018) A sex-structured model with birth pulse and release strategy for the spread of wolbachia in mosquito population. J Theor Biol 448:53–65
McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang Y-F, O’neill SL (2009) Stable introduction of a life-shortening wolbachia infection into the mosquito aedes aegypti. Science 323(5910):141–144
Ndii MZ, Hickson RI, Allingham D, Mercer GN (2015) Modelling the transmission dynamics of dengue in the presence of wolbachia. Math Biosci 262:157–166
O’Connor L, Plichart C, Sang AC, Brelsfoard CL, Bossin HC, Dobson SL (2012) Open release of male mosquitoes infected with a wolbachia biopesticide: field performance and infection containment. PLoS Neglect Trop D 6(11):e1797
Perlmutter JI, Meyers JE, Bordenstein SR (2020) Transgenic testing does not support a role for additional candidate genes in wolbachia male killing or cytoplasmic incompatibility. mSystems 5(1):1–20
Rasgon JL, Styer LM, Scott TW (2003) Wolbachia-induced mortality as a mechanism to modulate pathogen transmission by vector arthropods. J Med Entomol 40(2):125–132
Ritchie SA, van den Hurk AF, Smout MJ, Staunton KM, Hoffmann AA (2018) Mission accomplished? we need a guide to the ’post release’ world of wolbachia for aedes-borne disease control. Trends Parasitol 34(3):217–226
Segoli M, Hoffmann AA, Lloyd J, Omodei GJ, Ritchie SA (2014) The effect of virus-blocking wolbachia on male competitiveness of the dengue vector mosquito, aedes aegypti. PLoS Neglect Trop D 8(12):e3294
Shan C, Zhu H (2014) Bifurcations and complex dynamics of an sir model with the impact of the number of hospital beds. J Differ Equ. 257(5):1662–1688
Tolle MA (2009) Mosquito-borne diseases. Curr Prob Pediatr Adolesc Health Care 39:97–140
Turelli M (1994) Evolution of incompatibility-inducing microbes and their hosts. Evolution 48:1500–1513
Turelli M, Hoffmann AA (1991) Rapid spread of an inherited incompatibility factor in california drosophila. Nature 353(6343):440
Vautrin E, Charles S, Genieys S, Vavre F (2007) Evolution and invasion dynamics of multiple infections with wolbachia investigated using matrix based models. J Theor Biol 245:197–209
Walker TJPH, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P et al (2011) The wmel wolbachia strain blocks dengue and invades caged aedes aegypti populations. Nature 476:450
Waltz E (2016) Infected mosquitoes could fight zika. Nature 533:22–25
Waltz E (2017) USA government approves ‘killer’ mosquitoes to fight disease. https://www.nature.com/news/us-government-approves-killer-mosquitoes-to- fight-disease-1.22959. Accessed 06 Nov 2017
Wang Y, Pons W, Fang J, Zhu H (2017) The impact of weather and storm water management ponds on the transmission of west nile virus. R Soc Open Sci 4(8):170017
Wiggins S (2003) Introduction to applied nonlinear dynamical systems and chaos, vol 2. Springer, New York
Yang HM, Macoris MLG, Galvani KC, Andrighetti MTM, Wanderley DMV (2009) Assessing the effects of temperature on the population of aedes aegypti, the vector of dengue. Epidemiol Infect 137:1188–1202
Xianghong Z, Tang S, Cheke RA, Zhu H (2016) Modeling the effects of augmentation strategies on the control of dengue fever with an impulsive differential equation. B Math Biol 78:1968–2010
Zhang X, Tang S, Liu Q, Cheke RA, Zhu H (2018) Models to assess the effects of non-identical sex ratio augmentations of wolbachia-carrying mosquitoes on the control of dengue disease. Math Biosci 299:58–72
Zheng B, Tang M, Yu J (2014) Modeling wolbachia spread in mosquitoes through delay differential equations. SIAM J Appl Math 74:743–770
Zheng X, Zhang D, Li Y, Yang C, Wu Y, Liang X, Liang Y, Pan X, Hu L, Sun Q et al (2019) Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 572(7767):56–61
Acknowledgements
This work is supported by the National Natural Science Foundation of China (NSFC, 61772017), the Doctoral Fund of Southwest University (No. SWU019046) and the NSERC and CIHR of Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, X., Liu, Q. & Zhu, H. Modeling and dynamics of Wolbachia-infected male releases and mating competition on mosquito control. J. Math. Biol. 81, 243–276 (2020). https://doi.org/10.1007/s00285-020-01509-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-020-01509-7
Keywords
- Mosquito-borne diseases (MBDs)
- Wolbachia
- Mosquito suppression
- Mating competition
- Backward bifurcation
- Global stability