Abstract
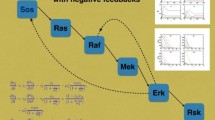
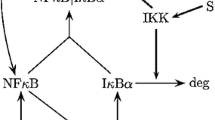
The MAPK signaling cascade is nowadays understood as a network module highly conserved across species. Its main function is to transfer a signal arriving at the plasma membrane to the cellular interior. Current understanding of ‘how’ this is achieved involves the notions of ultrasensitivity and bistability which relate to the nonlinear dynamics of the biochemical network, ignoring spatial aspects. Much less, indeed, is so far known about the propagation of the signal through the cytoplasm. In this work we formulate, starting from a Michaelis–Menten model for the MAPK cascade in Xenopus oocytes, a reaction-diffusion model of the cascade. We study this model in one space dimension. Basing ourselves on previous general results on reaction diffusion models, we particularly study for our model the conditions for signal propagation. We show that the existence of a propagating front depends sensitively on the initial and boundary conditions at the plasma membrane. Possible biological consequences of this finding are discussed.
Similar content being viewed by others
References
Amiel A, Leclère L, Robert L, Chevalier S, Houliston E (2009) Conserved functions for Mos in eumetazoan oocyte maturation revealed by studies in a cnidarian. Curr Biol 19: 305–311
Angeli D, Ferrell JE Jr, Sontag ED (2004) Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feedback systems. Proc Natl Acad Sci USA 101(7): 1822–1827
Aronson DG, Weinberger HF (1975) Nonlinear diffusion in population genetics, combustion, and nerve pulse propagation. In: Partial differential equations and related topics (Program, Tulane Univ, New Orleans, La, 1974). Lecture Notes in Math. vol 446. Springer, Berlin, pp 5–49
Baert F, Bodart J-F, Bocquet-Muchembled B, Lescuyer-Rousseau A, Vilain J-P (2003) Xp42(Mpk1) activation is not required for germinal vesicle breakdown but for Raf complete phosphorylation in insulin-stimulated Xenopus oocytes. J Biol Chem 278: 49714–49720
Brown MD, Sacks DB (2009) Protein scaffolds in MAP kinase signalling. Cell Signal 21: 462–469
Capco D-G, Bement W-M (1991) Analysis of cellular signaling events, the cytoskeleton, and spatial organization of macromolecules during early Xenopus development. Methods Cell Biol 36: 249–270
Castro A, Peter M, Lorca T, Mandart E (2001) c-Mos and cyclinB/cdc2 connections during Xenopus oocyte maturation. Mol Biol Cell 93: 15–25
Dupré A, Jessus C, Ozon R, Haccard O (2002) Mos is not required for the initiation of meiotic maturation in Xenopus oocytes. EMBO J 21: 4026–4036
Ferrell JE Jr, Pomerening JR, Kim SY, Trunnell NB, Xiong W, Huang CY, Machleder EM (2009) Simple, realistic models of complex biological processes: positive feedback and bistability in a cell fate switch and cell cycle oscillator. FEBS Lett 583: 3999–4005
Ferrell JE Jr, Machleder EM (1998) The biochemical basis for an all-or-none cell fate switch in Xenopus oocytes. Science 280: 895–898
Goldbeter A, Koshland DE (1981) An amplified sensitivity arising from covalent modification in biological systems. Proc Natl Acad Sci USA 78(11): 6840–6844
Groisman I, Huang YS, Mendez R, Cao Q, Theurkauf W, Richter J-D (2000) CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell 103: 435–447
Howard EL, Charlesworth A, Welk J, MacNicol AM (1999) The mitogen-activated protein kinase signaling pathway stimulates mos mRNA cytoplasmic polyadenylation during Xenopus oocyte maturation. Mol Cell Biol 161: 1990–1999
Huang CY, Ferrell JE Jr (1996) Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA 93: 10078–10083
Keady BT, Kuo P, Martinez SE, Yuan L, Hake LE (2007) MAPK interacts with XGEF and is required for CPEB activation during meiosis in Xenopus oocytes. J Cell Sci 19: 1093–1103
Kholodenko BN (2000) Negative feedback and ultrasensitivity can bring about oscillations in the mitogen-activated protein kinase cascades. Eur J Biochem 267(6): 1583–1588
Kholodenko BN (2002) MAP kinase cascade signaling and endocytic trafficking: a marriage of convenience?. Trends Cell Biol 12: 173–177
Kholodenko BN (2006) Cell-signalling dynamics in time and space. Nat Rev Mol Cell Biol 7: 165–176
Kholodenko BN, Hancock JF, Kolch W (2010) Signalling ballet in space and time. Nat Rev Mol Cell Biol 11: 414–426
Markevich NI, Tsyganov MA, Hoek JB, Kholodenko BN (2006) Long-range signaling by phosphoprotein waves arising from bistability in protein kinase cascade. Mol Syst Biol (article number: 61)
Matten WT, Copeland TD, Ahn NG, Van de Woude GF (1996) Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev Biol 179: 485–492
Morgan DO (2007) The cell cycle: principles of control. New Science Press, UK
Muoz-Garca J, Neufeld Z, Kholodenko BN (2009) Positional information generated by spatially distributed signaling cascades. PLoS Comp Biol 5(3:e1000330)
Murray JD (1993) Mathematical biology. In: Biomathematics, vol 19, 2nd edn. Springer, New York
Nebreda AR, Gannon JV, Hunt T (1995) Newly synthesized proteins must associate with p34-cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J 14: 5597–5607
Russo C, Beaujois R, Bodart J-F, Blossey R (2009) Kicked by Mos and tuned by MPF-the initiation of the MAPK cascade in Xenopus oocytes. HFSP J 3: 428–440
Russo C, Giuraniuc C, Blossey R, Bodart J-F (2009) On the equilibria of the mapk cascade: Cooperativity, modularity and bistability. Phys A Stat Mech Appl 388(24): 5070–5080
Santos SD, Verveer PJ, Bastiaens PI (2007) Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat Cell Biol 9: 324–330
Sheng J, Kumagai A, Dunphy W, Varshavsky A (2002) Dissection of c-mos degron. EMBO J 21: 6061–6071
Suzuki H, Tsukahara T, Inoue K (2009) Localization of c-mos mRNA around the animal pole in the zebrafish oocyte with Zor-1/Zorba. Biosci Trends 3: 96–104
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blossey, R., Bodart, JF., Devys, A. et al. Signal propagation of the MAPK cascade in Xenopus oocytes: role of bistability and ultrasensitivity for a mixed problem. J. Math. Biol. 64, 1–39 (2012). https://doi.org/10.1007/s00285-011-0403-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-011-0403-y