Abstract
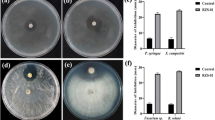
Duckweed-associated actinobacteria are co-existing microbes that affect duckweed growth and adaptation. In this study, we aimed to report a novel actinobacterium species and explore its ability to enhance duckweed growth. Strain DW7H6T was isolated from duckweed, Lemna aequinoctialis. Phylogenetic analysis based on its 16S rRNA gene sequence revealed that the strain was most closely related to Actinomycetospora straminea IY07-55T (99.0%), Actinomycetospora chibensis TT04-21T (98.9%), Actinomycetospora lutea TT00-04T (98.8%) and Actinomycetospora callitridis CAP 335T (98.4%). Chemotaxonomic and morphological characteristics of strain DW7H6T were consistent with members of the genus Actinomycetospora, while average nucleotide identity (ANI) and digital DNA-DNA hybridization (dDDH) between the draft genomes of this strain and its closely related type strains were below the proposed threshold values used for species discrimination. Based on chemotaxonomic, phylogenetic, phenotypic, and genomic evidence obtained, we describe a novel Actinomycetospora species, for which the name Actinomycetospora lemnae sp. nov. is proposed. The type strain is DW7H6T (TBRC 15165T, NBRC 115294T). Additionally, the duckweed-associated actinobacterium strain DW7H6T was able to enhance duckweed growth when compared to the control, in which the number of fronds and biomass dry weight were increased by up to 1.4 and 1.3 fold, respectively. Moreover, several plant-associated gene features in the genome of strain DW7H6T potentially involved in plant-microbe interactions were identified.



Similar content being viewed by others
Abbreviations
- ANI:
-
Average nucleotide identity
- dDDH:
-
Digital DNA-DNA hybridization
- GGDC:
-
Genome-to-Genome Distance Calculator
References
Bog M, Appenroth KJ, Sree KS (2019) Duckweed (Lemnaceae): its molecular taxonomy. Front Sustain Food Syst 3:117. https://doi.org/10.3389/fsufs.2019.00117
Cui W, Cheng JJ (2015) Growing duckweed for biofuel production: a review. Plant Biol 17(Suppl 1):16–23. https://doi.org/10.1111/plb.12216
Zeller MA, Hunt R, Sharma S (2013) Sustainable bioderived polymeric materials and thermoplastic blends made from floating aquatic macrophytes such as duckweed. J Appl Polym Sci 127(1):375–386. https://doi.org/10.1002/app.37555
Goopy JP, Murray PJ (2003) A review on the role of duckweed in nutrient reclamation and as a source of animal feed. Asian-Australas J Anim Sci 16(2):297–305. https://doi.org/10.5713/ajas.2003.297
Appenroth KJ, Sree KS, Bog M, Ecker J, Seeliger C, Böhm V, Lorkowski S, Sommer K, Vetter W, Tolzin-Banasch K, Kirmse R, Leiterer M, Dawczynski C, Liebisch G, Jahreis G (2018) Nutritional value of the duckweed species of the genus Wolffia (Lemnaceae) as human food. Front Chem. https://doi.org/10.3389/fchem.2018.00483
Cheng JJ, Stomp AM (2009) Growing duckweed to recover nutrients from wastewaters and for production of fuel ethanol and animal feed. Clean Soil Air Water 37(1):17–26. https://doi.org/10.1002/clen.200800210
El-Shafai SA, El-Gohary FA, Nasr FA, van der Peter N, Gijzen HJ (2007) Nutrient recovery from domestic wastewater using a UASB-duckweed ponds system. Bioresour Technol 98(4):798–807. https://doi.org/10.1016/j.biortech.2006.03.011
Ishizawa H, Kuroda M, Morikawa M, Ike M (2017) Evaluation of environmental bacterial communities as a factor affecting the growth of duckweed Lemna minor. Biotechnol Biofuels 10:62. https://doi.org/10.1186/s13068-017-0746-8
Khairina Y, Jog R, Boonmak C, Toyama T, Oyama T, Morikawa M (2021) Indigenous bacteria, an excellent reservoir of functional plant growth promoters for enhancing duckweed biomass yield on site. Chemosphere 268:129247.
Suzuki W, Sugawara M, Miwa K, Morikawa M (2014) Plant growth-promoting bacterium Acinetobacter calcoaceticus P23 increases the chlorophyll content of the monocot Lemna minor (duckweed) and the dicot Lactuca sativa (lettuce). J Biosci Bioeng 118(1):41–44. https://doi.org/10.1016/j.jbiosc.2013.12.007
Acosta K, Xu J, Gilbert S, Denison E, Brinkman T, Lebeis S, Lam E (2020) Duckweed hosts a taxonomically similar bacterial assemblage as the terrestrial leaf microbiome. PLoS ONE. https://doi.org/10.1371/journal.pone.0228560
Bunyoo C, Roongsattham P, Khumwan S, Phonmakham J, Wonnapinij P, Thamchaipenet A (2022) Dynamic alteration of microbial communities of duckweeds from nature to nutrient-deficient condition. Plants 11(21):2915. https://doi.org/10.3390/plants11212915
Huang W, Gilbert S, Poulev A, Acosta K, Lebeis S, Long C, Lam E (2020) Host-specific and tissue-dependent orchestration of microbiome community structure in traditional rice paddy ecosystems. Plant Soil 452(1):379–395. https://doi.org/10.1007/s11104-020-04568-3
Kittiwongwattana C, Vuttipongchaikij S (2015) Biodiversity of endophytic bacteria isolated from duckweed (Landoltia punctata) and their IAA production. Thammasat Int J Sci Tech 20(1):1–11
Saimee Y, Duangmal K (2021) Streptomyces spirodelae sp. nov., isolated from duckweed. Int J Syst Evol 71(11):005106. https://doi.org/10.1099/ijsem.0.005106
Parte AC, Sardà Carbasse J, Meier-Kolthoff JP, Reimer LC, Göker M (2020) List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol 70(11):5607–5612. https://doi.org/10.1099/ijsem.0.004332
Küster E, Williams ST (1964) Selection of media for isolation of streptomycetes. Nature 202(4935):928–929. https://doi.org/10.1038/202928a0
Waksman SA (1950) The actinomycetes: their nature, occurrence, activities, and importance. Chronica Botanica Company, Waltham, Massachusetts
Kieser T, Bibb MJ, Chater KF, Butter MJ, Hopwood DA (2000) Practical Streptomyces Genetics. John Innes Foundation, Norwich
Himaman W, Thamchaipenet A, Pathom-aree W, Duangmal K (2016) Actinomycetes from Eucalyptus and their biological activities for controlling Eucalyptus leaf and shoot blight. Microbiol Res 188–189:42–52. https://doi.org/10.1016/j.micres.2016.04.011
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67(5):1613–1617. https://doi.org/10.1099/ijsem.0.001755
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17(6):368–376. https://doi.org/10.1007/BF01734359
Fitch WM (1971) Toward defining the course of evolution: minimum change for a specific tree topology. Syst Biol 20(4):406–416. https://doi.org/10.1093/sysbio/20.4.406
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. PNAS 101(30):11030–11035. https://doi.org/10.1073/pnas.040420610
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. J Mol Evol 39(4):783–791. https://doi.org/10.2307/2408678
Shirling ET, Gottlieb D (1966) Methods for characterization of Streptomyces species1. Int J Syst Bacteriol 16(3):313–340. https://doi.org/10.1099/00207713-16-3-313
Mundie D (1995) The NBS/ISCC Color System/David A. Mundie, vol 5356 dc–20. Polymath Systems, Pittsburgh, PA
Gordon MM (1947) The concept of the sub-culture and its application. Soc Forces 26(1):40–42. https://doi.org/10.2307/2572602
Williams ST, Goodfellow M, Alderson G, Wellington EM, Sneath PH, Sackin MJ (1983) Numerical classification of Streptomyces and related genera. J Gen Microbiol 129(6):1743–1813. https://doi.org/10.1099/00221287-129-6-1743
Gordon RE, Mihm JM (1957) A comparative study of some strains received as nocardiae. J Bacteriol 73(1):15–27. https://doi.org/10.1128/jb.73.1.15-27.1957
Becker B, Lechevalier MP, Lechevalier HA (1965) Chemical composition of cell-wall preparations from strains of various form-genera of aerobic actinomycetes. Appl Microbiol 13(2):236–243. https://doi.org/10.1128/am.13.2.236-243.1965
Hasegawa T, Takizawa M, Tanida S (1983) A rapid analysis for chemical grouping of aerobic actinomycetes. J Gen Appl Microbiol 29(4):319–322. https://doi.org/10.2323/jgam.29.319
Staneck JL, Roberts GD (1974) Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl Microbiol 28(2):226–231
Tomiyasu I (1982) Mycolic acid composition and thermally adaptative changes in Nocardia asteroides. J Bacteriol 151(2):828–837
Minnikin DE, Patel PV, Alshamaony L, Goodfellow M (1977) Polar lipid composition in the classification of Nocardia and related bacteria. Int J Syst Evol Microbiol 27(2):104–117. https://doi.org/10.1099/00207713-27-2-104
Tindall BJ (1990) Lipid composition of Halobacterium lacusprofundi. FEMS Microbiol Lett 66(1–3):199–202. https://doi.org/10.1111/j.1574-6968.1990.tb03996.x
Tindall BJ (1990) A comparative study of the lipid composition of Halobacterium saccharovorum from various sources. Syst Appl Microbiol 13(2):128–130. https://doi.org/10.1016/S0723-2020(11)80158-X
Sasser M (1990) Identification of bacteria by gas chromatography of cellular fatty acids. MIDI technical note 101. MIDI inc, Newark, DE
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19(5):455–477. https://doi.org/10.1089/cmb.2012.0021
Mikheenko A, Prjibelski A, Saveliev V, Antipov D, Gurevich A (2018) Versatile genome assembly evaluation with QUAST-LG. Bioinform 34(13):i142–i150
Meier-Kolthoff JP, Carbasse JS, Peinado-Olarte RL, Göker M (2022) TYGS and LPSN: a database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res 50(D1):D801–d807. https://doi.org/10.1093/nar/gkab902
Meier-Kolthoff JP, Göker M (2019) TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun 10(1):1–10. https://doi.org/10.1038/s41467-019-10210-3
Richter M, Rosselló-Móra R, Oliver Glöckner F, Peplies J (2016) JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinform 32(6):929–931. https://doi.org/10.1093/bioinformatics/btv681
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O (2008) The RAST server: Rapid annotations using Subsystems Technology. BMC Genomics 9(1):75. https://doi.org/10.1186/1471-2164-9-75
Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J (2016) NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 44(14):6614–6624. https://doi.org/10.1093/nar/gkw569
Blin K, Shaw S, Kloosterman AM, Charlop-Powers Z, Van Wezel GP, Medema MH, Weber T (2021) antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res 49(W1):W29–W35. https://doi.org/10.1093/nar/gkab335
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Analy Biochem 160(1):47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Gilbert S, Xu J, Acosta K, Poulev A, Lebeis S, Lam E (2018) Bacterial production of indole related compounds reveals their role in association between duckweeds and endophytes. Front Chem 6:265. https://doi.org/10.3389/fchem.2018.00265
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26(1):192–195. https://doi.org/10.1104/pp.26.1.192
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Jiang Y, Wiese J, Tang S-K, Xu L-H, Imhoff JF, Jiang C-L (2008) Actinomycetospora chiangmaiensis gen. nov., sp. nov., a new member of the family Pseudonocardiaceae. Int J Syst Evol Microbiol 58(2):408–413.
Tamura T, Ishida Y, Hamada M, Otoguro M, Yamamura H, Hayakawa M, Suzuki K-I (2011) Description of Actinomycetospora chibensis sp. nov., Actinomycetospora chlora sp. nov., Actinomycetospora cinnamomea sp. nov., Actinomycetospora corticicola sp. nov., Actinomycetospora lutea sp. nov., Actinomycetospora straminea sp. nov. and Actinomycetospora succinea sp. nov. and emended description of the genus Actinomycetospora. Int J Syst Evol 61(6):1275–1280. https://doi.org/10.1099/ijs.0.024166-0
Lechevalier MP, De Bievre C, Lechevalier H (1977) Chemotaxonomy of aerobic actinomycetes: phospholipid composition. Biochem Syst Ecol 5(4):249–260. https://doi.org/10.1016/0305-1978(77)90021-7
Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, da Costa MS, Rooney AP, Yi H, Xu X-W, De Meyer S, Trujillo ME (2018) Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol 68(1):461–466. https://doi.org/10.1099/ijsem.0.002516
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:963401. https://doi.org/10.6064/2012/963401
Vuori K-M (1995) Direct and indirect effects of iron on river ecosystems. Annales Zoologici Fennici. JSTOR, New York
Sinha AK, Parli Venkateswaran B, Tripathy SC, Sarkar A, Prabhakaran SJJ (2019) Effects of growth conditions on siderophore producing bacteria and siderophore production from Indian Ocean sector of Southern Ocean. J Basic Microbiol 59(4):412–424. https://doi.org/10.1002/jobm.201800537
Christina Jenifer A, Sharmili S, Anbumalarmathi J, Umamaheswari K, Shyamala K (2015) Studies on siderophore production by microbial isolates obtained from aquatic environment. Euro J Exp Bio 5(10):41–45
Yamakawa Y, Jog R, Morikawa M (2018) Effects of co-inoculation of two different plant growth-promoting bacteria on duckweed. Plant Growth Regul 86(2):287–296. https://doi.org/10.1007/s10725-018-0428-y
Yamaga F, Washio K, Morikawa M (2010) Sustainable biodegradation of phenol by Acinetobacter calcoaceticus P23 isolated from the rhizosphere of duckweed Lemna aoukikusa. Environ Sci Technol 44(16):6470–6474. https://doi.org/10.1021/es1007017
Ishizawa H, Kuroda M, Inoue K, Inoue D, Morikawa M, Ike M (2019) Colonization and competition dynamics of plant growth-promoting/inhibiting bacteria in the phytosphere of the duckweed Lemna minor. Microb Ecol 77(2):440–450. https://doi.org/10.1007/s00248-018-1306-x
Ishizawa H, Ogata Y, Hachiya Y, Tokura K-i, Kuroda M, Inoue D, Toyama T, Tanaka Y, Mori K, Morikawa M, Ike M (2020) Enhanced biomass production and nutrient removal capacity of duckweed via two-step cultivation process with a plant growth-promoting bacterium, Acinetobacter calcoaceticus P23. Chemosphere 238:124682. https://doi.org/10.1016/j.chemosphere.2019.124682
Ishizawa H, Kuroda M, Inoue D, Morikawa M, Ike M (2020) Community dynamics of duckweed-associated bacteria upon inoculation of plant growth-promoting bacteria. FEMS Microbiol Ecol 96(7):fiaa 101. https://doi.org/10.1093/femsec/fiaa101
Iwashita T, Tanaka Y, Tamaki H, Nakai R, Yoneda Y, Makino A, Toyama T, Kamagata Y, Morikawa M, Mori K (2021) Isolation and characterization of novel plant growth-promoting bacteria from the fronds of duckweed. Japanese J Water Treat Biology 57(1):1–9. https://doi.org/10.2521/jswtb.57.1
Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, Subramanian S, Smith DL (2018) Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 9:01473. https://doi.org/10.3389/fpls.2018.01473
Nazir N, Kamili AN, Shah D (2018) Mechanism of plant growth promoting rhizobacteria (PGPR) in enhancing plant growth-A review. Int J Manag Technol Eng 8:709–721
Vansuyt G, Robin A, Briat JF, Curie C, Lemanceau P (2007) Iron acquisition from Fe-pyoverdine by Arabidopsis thaliana Molecular. plant-microbe Interactions: MPMI 20(4):441–447. https://doi.org/10.1094/mpmi-20-4-0441
Kaewkla O, Franco CMM (2019) Actinomycetospora callitridis sp. nov., an endophytic actinobacterium isolated from the surface-sterilised root of an Australian native pine tree. Antonie van Leeuwenhoek 112(3):331–337.
Acknowledgements
We thank Professor Aharon Oren for his kind advice on naming the species. We also acknowledge a support from Science and Technology Research Partnership for Sustainable Development (SATREPS), JICA.
Funding
This work was financially supported by Kasetsart University Research and Development Institute (KURDI) under the project FF(KU)4.64.
Author information
Authors and Affiliations
Contributions
Yuparat Saimee: conceptualization, performed the experiment, formal analysis, writing–original draft; Waranya Butdee: performed partial experiment; Chanita Boonmak: critical review of manuscript; Kannika Duangmal: conceptualization, writing–review and editing, supervision, project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Approval
No animals or human participants were included in the present study.
Consent for publication
All the authors agree to submit for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The GenBank accession number for the 16S rRNA gene sequence of strain DW7H6T is OQ415442. The genome sequences of strain DW7H6T, Actinomycetospora straminae JCM 17983T, Actinomycetospora chibensis JCM 17987T, Actinomycetospora lutea TBRC 2215T, and DSM 101857T are JAQZAO000000000, JAQZAN000000000, JAQZAM000000000, JAQZAL000000000, JAQZAK000000000, respectively.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saimee, Y., Butdee, W., Boonmak, C. et al. Actinomycetospora lemnae sp. nov., A Novel Actinobacterium Isolated from Lemna aequinoctialis Able to Enhance Duckweed Growth. Curr Microbiol 81, 92 (2024). https://doi.org/10.1007/s00284-023-03595-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03595-4