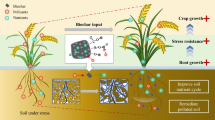
Abstract
Heavy metals are considered to be one of the main sources of soil contamination. In this study, three tolerant bacteria were isolated from the heavy metal-contaminated soil in mining area, and immobilized bacteria were constructed using corn straw as the carrier. The combined remediation effect of immobilized bacteria and alfalfa in pot experiments was explored in heavy metal-contaminated soil. Under heavy metal stress, inoculation with immobilized bacteria significantly promoted the growth of alfalfa, in which the dry weights of roots, stems, and leaves increased by 19.8, 6.89, and 14.6%, respectively (P < 0.05). Also, inoculation with immobilized bacteria improved the antioxidant capacity of plants and the activity of soil enzymes and improved soil quality (P < 0.05). Microbial-phytoremediation technology effectively reduced the heavy metal content in the soil, and can restore the soil contaminated by heavy metals. The results will help to further understand the mechanism of microbial inoculation to reduce the toxicity of heavy metals, and provide guidance for the cultivation of forage grasses in heavy metal-contaminated soils.
Graphical Abstract





Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Huang Q, Shindo H (2000) Effects of copper on the activity and kinetics of free and immobilized acid phosphatase. Soil Biol Biochem 32(13):1885–1892. https://doi.org/10.1016/S0038-0717%20(00)00162-0
Abd-Alla MH, Morsy FM, El-Enany AWE (2012) Ohyama T Isolation and characterization of a heavy-metal-resistant isolate of Rhizobium leguminosarum bv. viciae potentially applicable for biosorption of Cd2+ and Co2+. Int Biodeterior Biodegrad 67:48–55
Miralles J, Veron AJ, Radakovitch O, Deschamps P, Hamelin B (2006) Atmospheric lead fallout over the last century recorded in Gulf of Lions sediments (Mediterranean Sea). Mar Pollut Bull. 52(11):1364–1371. https://doi.org/10.1016/j.marpolbul.2006.03.018
Ojuederie OB, Babalola OO (2014) Microbial and plant-assisted bioremediation of heavy metalpolluted environments: a review. Int J Environ Res Public Health 14(12):1504. https://doi.org/10.3390/ijerph14121504
Karn SK, Pan X (2017) Bacterial oxidation and stabilization of As(III) in soil. Environ Eng Sci 34(3):158–164. https://doi.org/10.1089/ees.2015.0390
Chen YX, Wang YP, Lin Q, Luo YM (2005) Effect of copper-tolerant rhizosphere bacteria on mobility of copper in soil and copper accumulation by Elsholtzia splendens. Environ Int. 31(6):861–866. https://doi.org/10.1016/j.envint.2005.05.044
Bilal S, Shahzad R, Imran M, Jam R, Kim KM, Lee IJ (2020) Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: heavy metals, high temperature and drought stress. Ind Crops Prod 143:111931. https://doi.org/10.1016/j.indcrop.2019.111931
Yavari S, Malakahmad A, Sapari NB (2015) Biochar efficiency in pesticides sorption as a function of production variables-a review. Environ Sci Pollut Res 22(18):13824–13841. https://doi.org/10.1007/s11356-015-5114-2
Ab Aziz NS, Mohd Nor MA, Hamzah F (2015) Suitability of biochar produced from biomass waste as soil amendment. Proc Soc Behav Sci 195:2457–2465. https://doi.org/10.1016/j.sbspro.2015.06.288
Li Z, He F, Tong Z, Li X, Yan Q, Hannaway D (2022) Metabolomic changes in crown of alfalfa (Medicago sativa L.) duringde-acclimation. Sci Rep 12:14977. https://doi.org/10.21203/rs.3.rs-1515778/v1
Medina CA, Hawkins C, Liu XP, Peel M, Yu L (2020) Genome-wide association and prediction of traits related to salt tolerance in auto-tetraploid alfalfa (Medicago sativa L.). Int J Mol Sci 21(9):3361. https://doi.org/10.3390/ijms21093361%20
Liu Y (2022) Research on remediation of heavy metal contaminated tailings area soil by immobilized bacteria combined with herbaceous plants [D]. Lanzhou Jiaotong University. https://doi.org/10.27205/d.cnki.gltec.2022.000363
Kumar A, Singh S, Mukherjee A, Rastogi R, Verma J (2021) Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol Res 242:126616. https://doi.org/10.1016/j.micres.2020.126616
Takarina ND, Pin TG (2017) Bio-concentration factor (BCF) and translocation factor (TF) of heavy metals in mangrove trees of Blanakan fish farm. Makara J Sci. https://doi.org/10.7454/mss.v21i2.7308
Cui H, Yang X, Xu L, Fan Y, Yi Q, Li R, Zhou J (2017) Effects of goethite on the fractions of Cu, Cd, Pb, P and soil enzyme activity with hydroxyapatite in heavy metal-contaminated soil. RSC Adv 7:45869–45877. https://doi.org/10.1039/c7ra08786a
Gasco G, Paz-Ferreiro J, Cely P, Plaza C, Méndezd A (2016) Influence of pig manure and itsbiochar on soil CO2 emissions and soil enzymes. Ecol Eng 95:19–24. https://doi.org/10.1016/j.ecoleng.2016.06.039
Rizwan M, Ali S, Qayyum MF, Ibrahim M, Ziaurrehman M, Abbas T, Ok YS (2016) Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: a critical review. Environ Sci Pollut Res 23:2230–2248. https://doi.org/10.1007/s11356-015-5697-7
Qi W, Ling C, Linyan He et al (2016) Increased biomass and reduced heavy metal accumulation of edible tissues of vegetable crops in the presence of plant growth-promoting Neorhizobium huautlense T1–17 and biochar [J]. Agr Ecosyst Environ 228:9–18. https://doi.org/10.1016/j.agee.2016.05.006
Tu C, Wei J, Guan F, Liu Y, Sun Y, Luo Y (2020) Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ Int 137:105576. https://doi.org/10.1016/j.envint.2020.105576
Naveed M, Mustafa A, Majeed S, Naseem Z, Saeed Q, Khan A, Nawaz A, Baig K, Chen J (2020) Enhancing cadmium tolerance and pea plant health through Enterobacter sp MN17 inoculation together with biochar and gravel sand. Plants 9(4):530. https://doi.org/10.3390/plants9040530
Wu Y, Ma L, Liu Q, Vestergård M, Topalovic O, Wang Q, Feng Y (2020) The plant-growth promoting bacteria promote cadmium uptake by inducing a hormonal crosstalk and lateral root formation in a hyperaccumulator plant Sedum alfredii. J Hazard Mater 395:122661. https://doi.org/10.1016/j.jhazmat.2020.122661
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Yu F, Yao Y, Feng J, Wang X, Ma J, Liu K, Li Y (2021) FM-1 inoculation influenced heavy metal-induced oxidative stress in pakchoi (Brassica campestris L. ssp. Chinensis Makino) and water spinach (Ipomoea aquatic F.) cultivated in cadmium and lead co-contaminated soils. Plant Soil 459:155–171. https://doi.org/10.1007/s11104-020-04752-5
Grant JJ, Loake GJ (2000) Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol 124(1):21–30. https://doi.org/10.1104/pp.124.1.21
Willekens H, Chamnongpol S, Davey M, Davey M, Schraudner M, Langebartels C, Montagu M, Inze D, Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. The EMBO J 16(16):4806–4816. https://doi.org/10.1093/emboj/16.16.4806
Li Y, Liu K, Zhu J, Jiang Y, Huang Y, Zhou Z, Chen C, Yu F (2019) Manganese accumulation and plant physiology behavior of Camellia oleifera in response to different levels of nitrogen fertilization. Ecotoxicol Environ Saf 184:109603. https://doi.org/10.1016/j.ecoenv.2019.109603
Chatterjee P, Samaddar S, Anandham R, Kang YY, Kim K, Selvakumar G, Sa T (2017) Beneficial soil bacterium Pseudomonas frederiksbergensis OS261 augments salt tolerance and promotes red pepper plant growth. Front Plant Sci 8:705. https://doi.org/10.3389/fpls.2017.00705
Ma Y, Rajkumar M, Freitas H (2009) Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. Journal ofHazardous Materials 166:1154–1161. https://doi.org/10.1016/j.jhazmat.2008.12.018
Burd GI, Dixon DG, Glick BR (1998) A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl Environ Microbiol 64:3663–3668. https://doi.org/10.1128/AEM.64.10.3663-3668.1998
Sharma RK, Archana G (2016) Cadmium minimization in food crops by cadmium resistant plant growth promoting rhizobacteria. Appl Soil Ecol 107:66–78. https://doi.org/10.1016/j.apsoil.2016.05.009
Ma Y, Rajkumar M, Zhang C, Freitas H (2016) Beneficial role of bacterial endophytes in heavy metal phytoremediation. J Environ Manage 174:14–25. https://doi.org/10.1016/j.jenvman.2016.02.047
Etesami H (2018) Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: mechanisms and future prospects. Ecotoxicol Environ Saf 147:175–191. https://doi.org/10.1016/j.ecoenv.2017.08.032
Wang X, Cai D, Ji M, Chen Z, Yao L, Han H (2022) Isolation of heavy metal-immobilizing and plant growth-promoting bacteria and their potential in reducing Cd and Pb uptake in water spinach. Sci Total Environ 819:153242. https://doi.org/10.1016/j.scitotenv.2022.153242
Yoon J, Cao X, Zhou Q, Ma L (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368(2–3):456–464. https://doi.org/10.1016/j.scitotenv.2006.01.016
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals concepts and applications. Chemosphere 91(7):869–881. https://doi.org/10.1016/j.chemosphere.2013.01.075
Qi R, Li J, Lin Z, Li Z, Li Y, Yang X, Zhang J, Zhao B (2016) Temperature effects on soil organic carbon, soil labile organic carbon fractions, and soil enzyme activities under long-term fertilization regimes. Applied Soil Ecol 102:36–45. https://doi.org/10.1016/j.apsoil.2016.02.004
Nannipieri P, Ascher J, Ceccherini MT, Ceccherini MT, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54(4):655–670. https://doi.org/10.1046/j.1351-0754.2003.0556.x
Zhang Q, Zhu L, Wang J, Xie H, Wang J, Wang F, Sun F (2014) Effects of fomesafen on soil enzyme activity, microbial population, and bacterial community composition. Environ Monit Assess 186(5):2801–2812. https://doi.org/10.1007/s10661-013-3581-9
Ge T, Nie S, Wu J, Shen J, Xioa H, Tong C, Huang D, Hong Y, Iwasaki K (2011) Chemical properties, microbial biomass, and activity differ between soils of organic and conventional horticultural systems under greenhouse and open field management: a case study. J Soils Sediments 11(1):25–36. https://doi.org/10.1007/s11368-010-0293-4
Jia X, Zhong Y, Liu J, Zhu G, Shangguan Z, Yan W (2020) Effects of nitrogen enrichment on soil microbial characteristics: from biomass to enzyme activities. Geoderma 366:114256. https://doi.org/10.1016/j.geoderma.2020.114256
Aponte H, Meli P, Butler B, Paolini J, Matus F, Merino C, Kuzyakov Y (2020) Meta-analysis of heavy metal effects on soil enzyme activities. Sci Total Environ 737:139744. https://doi.org/10.1016/j.scitotenv.2020.139744
Lin H, Liu C, Li B, Dong Y (2021) Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J Hazard Mater 402:123829. https://doi.org/10.1016/j.jhazmat.2020.123829
Xu Z, Yang Z, Zhu T, Shu W, Geng L (2021) Ecological improvement of antimony and cadmium contaminated soil by earthworm Eisenia fetida: Soil enzyme and microorganism diversity. Chemosphere 273:129496. https://doi.org/10.1016/j.chemosphere.2020.129496
Li B, Dai Y, Song W, Pan X (2021) Effects of alfalfa root exudates on the microbial remediation of nitrate-heavy metal complex pollution in groundwater. Sci Asia 47:366. https://doi.org/10.2306/scienceasia1513-1874.2021.047
Ju W, Jin X, Liu L, Shen G, Zhao W, Duan C, Fang L (2020) Rhizobacteria inoculation benefits nutrient availability for phytostabilization in copper contaminated soil: drivers from bacterial community structures in rhizosphere. Appl. soil Ecol 150:103450. https://doi.org/10.1016/j.apsoil.2019.103450
Funding
This work was supported by the National Natural Science Foundation of China (Grant numbers NSFC 31860176, 32171611, and 32101415), Key Research and Development Program of Gansu Province (20YF3FA037, 22JR5RA454, and 22JR5RA262), Key Research and Development Program of Shanxi Province (2020ZDLSF06-06, 2021JQ-791, and XAWLKYTD012). We are grateful to all reviewers whose comments improved the quality of the manuscript.
Author information
Authors and Affiliations
Contributions
TG: Conceptualization, Methodology, Visualization, Investigation, Writing—original draft, and Writing—review & editing. YL: Formal analysis, Methodology, Writing—original draft, Writing—review & editing, and Investigation & editing. DY: Methodology, Investigation, and Writing—original draft. XL: Methodology and Investigation. MZ: Conceptualization and Validation. YH: Writing—original draft and Validation. HW: Writing—original draft, Writing—review & editing, and Supervision. JB: Validation, Visualization, Writing—Original Draft, and Formal analysis. YS: Validation. XT: Validation. DX: Methodology and Formal analysis. JX: Validation. All authors read and approved the final manuscript. All of the authors have read and approved the manuscript. This work has not been published previously, nor it is being considered by any other peer-reviewed journal.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
284_2023_3299_MOESM2_ESM.tif
Supplementary file2 (TIF 271 kb)—Supplementary Figure 2 Growth status of strains L1, L2 and L3 on the solid medium (Left: L1; Middle: L2; Light: L3)
284_2023_3299_MOESM3_ESM.tif
Supplementary file3 (TIF 4024 kb)—Supplementary Figure 3 Phylogenetic tree of three resistant bacteria (a: L1, b: L2, and c: L3)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, T., Liu, Y., Yang, D. et al. Inoculation of Exogenous Complex Bacteria to Enhance Resistance in Alfalfa and Combined Remediation of Heavy Metal-Contaminated Soil. Curr Microbiol 80, 213 (2023). https://doi.org/10.1007/s00284-023-03299-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03299-9