Abstract
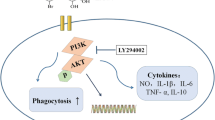
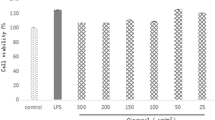
The purpose of this study was to discuss the effects of an extract from the culture medium of Pseudomonas aeruginosa (P. aeruginosa) 2016NX1 (chloroform extract of P. aeruginosa, CEPA) and its purified product 1-hydroxyphenazine on RAW264.7 cell inflammation. Cell viability was evaluated by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) method. TNF-α production was determined by an ELISA method. The effects of CEPA and its purified product 1-hydroxyphenazine on cell morphology were investigated using an inverted microscope. Quantitative real-time PCR was performed to determine mRNA expression levels. CEPA and 1-hydroxyphenazine had no obvious toxicity to cells when their concentrations were no more than 20 μg ml−1 and 5 μg ml−1, respectively. Both CEPA and 1-hydroxyphenazine suppressed the secretion of TNF-α and significantly reduced the mRNA expression levels of TNF-α, IL-1β, and IL-6. Both CEPA and 1-hydroxyphenazine inhibited M1 cell polarization after lipopolysaccharide (LPS) stimulation. The results in this article lay a good foundation for the biopharmaceutical applications of CEPA and 1-hydroxyphenazine in the future. CEPA and 1-hydroxyphenazine had certain anti-inflammatory activity, and inhibited LPS-induced RAW264.7 cell inflammation. Our findings suggest that CEPA and 1-hydroxyphenazine are potential chemicals with anti-inflammatory activity.





Similar content being viewed by others
References
Ding Y, Dai L, Yu J (2020) Progress of research on treatment of Pseudomonas aeruginosa infection. Chin J Nosocomiol 30:955–960
Botelho J, Grosso F, Peixe L (2019) Antibiotic resistance in Pseudomonas aeruginosa-mechanisms, epidemiology and evolution. Drug Resist Updat 44:100640
Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z (2019) Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 37:177–192
Kariminik A, Baseri-Salehi M, Kheirkhah B (2017) Pseudomonas aeruginosa quorum sensing modulates immune responses: an updated review article. Immunol Lett 190:1–6
Khan R, Basha A, Goverdhanam R, Rao PC, Tanemura Y, Fujimoto Y, Begum AS (2015) Attenuation of TNF-alpha secretion by L-proline-based cyclic dipeptides produced by culture broth of Pseudomonas aeruginosa. Bioorg Med Chem Lett 25:5756–5761
Xiao M, Ruan C, Chen S, Liu Y, Lu Z (2018) Isolation and identification of a bacterium producing natural blue pigment. J Guangxi Norm Univ (Nat Sci Edit) 36:131–138
Xiao M, Sun M, Ruan C, Chen S, Liu Y, Lu Z (2019) Inhibitory effect of biocontrol bacterium 2016NX1 on plant pathogenic fungi and optimization of fermentation conditions. J Guangxi Norm Univ (Nat Sci Edit) 37:168–178
Liu TT, Ye FC, Pang CP, Yong TQ, Tang WD, Xiao J, Shang CH, Lu ZJ (2020) Isolation and identification of bioactive substance 1-hydroxyphenazine from Pseudomonas aeruginosa and its antimicrobial activity. Lett Appl Microbiol 71:303–310
McFarland AJ, Anoopkumar-Dukie S, Perkins AV, Davey AK, Grant GD (2012) Inhibition of autophagy by 3-methyladenine protects 1321N1 astrocytoma cells against pyocyanin- and 1-hydroxyphenazine-induced toxicity. Arch Toxicol 86:275–284
Kamal A, Shaik AB, Kumar CG, Mongolla P, Rani PU, Krishna KV, Mamidyala SK, Joseph J (2012) Metabolic profiling and biological activities of bioactive compounds produced by Pseudomonas sp. strain ICTB-745 isolated from Ladakh. India J Microbiol Biotechnol 22:69–79
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428–435
Messay B, Lim A, Marsland AL (2012) Current understanding of the bi-directional relationship of major depression with inflammation. Biol Mood Anxiety Disord 2:4
Kim KS, Cui X, Lee DS, Sohn JH, Yim JH, Kim YC, Oh H (2013) Anti-inflammatory effect of neoechinulin a from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-кB and p38 MAPK pathways in lipopolysaccharide-stimulated RAW264.7 macrophages. Molecules 18:13245–13259
Van Dyken SJ, Locksley RM (2013) Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol 31:317–343
Lim KH, Staudt LM (2013) Toll-like receptor signaling. Cold Spring Harb Perspect Biol 5:a011247
Czock D, Keller F, Rasche FM, Häussler U (2005) Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 44:61–98
Fernandes ACF, Vieira NC, Santana ÁL, Gandra RLP, Rubia C, Castro-Gamboa I, Macedo JA, Macedo GA (2020) Peanut skin polyphenols inhibit toxicity induced by advanced glycation end-products in RAW264.7 macrophages. Food Chem Toxicol 145:111619
Zhu L, Zhao Q, Yang T, Ding W, Zhao Y (2015) Cellular metabolism and macrophage functional polarization. Int Rev Immunol 34:82–100
Linton MF, Fazio S (2003) Macrophages, inflammation, and atherosclerosis. Int J Obes Relat Metab Disord 27:S35–S40
Holden JA, Attard TJ, Laughton KM, Mansell A, O’Brien-Simpson NM, Reynolds EC (2014) Porphyromonas gingivalis lipopolysaccharide weakly activates M1 and M2 polarized mouse macrophages but induces inflammatory cytokines. Infect Immun 82:4190–4203
O’Brien-Simpson NM, Pathirana RD, Walker GD, Reynolds EC (2009) Porphyromonas gingivalis RgpA-Kgp proteinase-adhesin complexes penetrate gingival tissue and induce proinflammatory cytokines or apoptosis in a concentration-dependent manner. Infect Immun 77:1246–1261
Yan Y, He YY, Fang LH, Du GH (2014) Research progress of the roles of macrophages in atherosclerosis. Chin Pharm J 49:7–10
Coward WR, Okayama Y, Sagara H, Wilson SJ, Holgate ST, Church MK (2002) NF-kappa B and TNF-alpha: a positive autocrine loop in human lung mast cells? J Immunol 169:5287–5293
Russo C, Polosa R (2005) TNF-alpha as a promising therapeutic target in chronic asthma: a lesson from rheumatoid arthritis. Clin Sci (Lond) 109:135–142
Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G (2012) Atherosclerosis as an inflammatory disease. Curr Pharm Des 18:4266–4288
Aggarwal BB (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3:745–756
Liu J, Marino MW, Wong G, Grail D, Dunn A, Bettadapura J, Slavin AJ, Old L, Bernard CC (1998) TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat Med 4:78–83
Song S, Xin P (2017) Effect of IL-1β, IL-6, TNF-α and IL-8 on the growth and metastasis of lung cancer in chronic obstructive pulmonary disease. J Clin Pathol Res 37:2323–2331
Li C, Guo Q (2018) Changes of serum IL-1β, IL-2, IL-6, IL-8 and TNF-α levels in patients with epilepsy and clinical significance. J Prev Med Chin People’s Lib Army 36:375–377
Hwang JH, Kim KJ, Ryu SJ, Lee BY (2016) Caffeine prevents LPS-induced inflammatory responses in RAW264.7 cells and zebrafish. Chem Biol Interact 248:1–7
Nemudzivhadi V, Masoko P (2014) In vitro assessment of cytotoxicity, antioxidant, and anti-inflammatory activities of Ricinus communis (Euphorbiaceae) leaf extracts. Evid Based Complement Alternat Med 2014:625961
Hwang D, Kang MJ, Kang CW, Kim GD (2019) Kaempferol-3-O-β-rutinoside suppresses the inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells via the NF-κB and MAPK pathways. Int J Mol Med 44:2321–2328
Chin-A-Woeng TFC, Bloemberg GV, Lugtenberg BJJ (2003) Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol 157:503–523
Jiang HX, Zhou L, He YW (2015) Research progress in biocontrol strain Pseudomonas aeruginosa: antifungal metabolites and their applications in biocontrol. Microbiol China 42:1338–1349
Fang YL, Sun S, Shen Y, He YW (2014) Progress on the development and application of biopesticide Shenqinmycin. Chin J Pestic Sci 16:387–393
Lin YW, Lee B, Liu PS, Wei LN (2016) Receptor-interacting protein 140 orchestrates the dynamics of macrophage M1/M2 polarization. J Innate Immun 8:97–107
Luo W, Ai L, Li X, Wang B, Zhou Y (2019) Chronic high glucose inhibits AKT phosphorylation and promotes M1 polarization of mouse RAW264.7 macrophages. Chin J Cell Mol Immunol 35:910–917
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A (2018) Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233:6425–6440
Wang SX, Zhang JM, Yao XM, Wang ZG, Mao XD, Cao M (2017) Effects of luteolin on the secretion of inflammatory cytokines from activated RAW264.7 macrophages. J Med Postgrad 30:31–35
Hu JJ, Zhang DD, Chen JJ, Chen CS, Li YP (2012) Effect of pretreatment with puerarin on activation of LPS-induced RAW264.7 cells. China J Chin Mater Med 37:3112–3116
Jung HW, Seo UK, Kim JH, Leem KH, Park YK (2009) Flower extract of Panax notoginseng attenuates lipopolysaccharide-induced inflammatory response via blocking of NF-kappaB signaling pathway in murine macrophages. J Ethnopharmacol 122:313–319
Cho W, Nam JW, Kang HJ, Windono T, Seo EK, Lee KT (2009) Zedoarondiol isolated from the rhizoma of Curcuma heyneana is involved in the inhibition of iNOS, COX-2 and pro-inflammatory cytokines via the downregulation of NF-kappaB pathway in LPS-stimulated murine macrophages. Int Immunopharmacol 9:1049–1057
Yun KJ, Shin JS, Choi JH, Back NI, Chung HG, Lee KT (2009) Quaternary alkaloid, pseudocoptisine isolated from tubers of Corydalis turtschaninovi inhibits LPS-induced nitric oxide, PGE(2), and pro-inflammatory cytokines production via the down-regulation of NF-kappaB in RAW 264.7 murine macrophage cells. Int Immunopharmacol 9:1323–1331
Zhang J, Gong F, Li L, Zhao M, Song J (2014) Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone attenuates lipopolysaccharide-induced inflammation by activating the unfolded protein response. Biomed Rep 2:233–238
Zhou W, Feng X, Xiao C, Li S, Wang C (2013) Role of BPIFB1 in regulating inflammatory response of RAW264.7 cells infected by P. aeruginosa. Chin J Cell Mol Immunol 29:602–605
Dharni S, Alam M, Kalani K, Abdul-Khaliq SA, Srivastava SK, Patra DD (2012) Production, purification, and characterization of antifungal metabolite from Pseudomonas aeruginosa SD12, a new strain obtained from tannery waste polluted soil. J Microbiol Biotechnol 22:674–683
Kerr JR, Taylor GW, Rutman A, Høiby N, Cole PJ, Wilson R (1999) Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J Clin Pathol 52:385–387
Qi X, Xue M, Cui H, Yang K, Song K, Zha J, Wang G, Ling F (2020) Antimicrobial activity of Pseudomonas monteilii JK-1 isolated from fish gut and its major metabolite, 1-hydroxyphenazine, against Aeromonas hydrophila. Aquaculture 526:735366
Prabhu MS, Walawalkar YD, Furtado I (2014) Purification and molecular and biological characterisation of the 1-hydroxyphenazine, produced by an environmental strain of Pseudomonas aeruginosa. World J Microbiol Biotechnol 30:3091–3099
Pierson LS 3rd, Pierson EA (2010) Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes. Appl Microbiol Biotechnol 86:1659–1670
Morales DK, Jacobs NJ, Rajamani S, Krishnamurthy M, Cubillos-Ruiz JR, Hogan DA (2010) Antifungal mechanisms by which a novel Pseudomonas aeruginosa phenazine toxin kills Candida albicans in biofilms. Mol Microbiol 78:1379–1392
Guttenberger N, Blankenfeldt W, Breinbauer R (2017) Recent developments in the isolation, biological function, biosynthesis, and synthesis of phenazine natural products. Bioorg Med Chem 25:6149–6166
Jobson AG, Willmore E, Tilby MJ, Mistry P, Charlton P, Austin CA (2009) Effect of phenazine compounds XR11576 and XR5944 on DNA topoisomerases. Cancer Chemother Pharmacol 63:889–901
Laursen JB, Nielsen J (2004) Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. Chem Rev 104:1663–1686
Zhu X, Zeng Y, Zhao X, Zou S, He YW, Liang Y (2017) A genetic screen in combination with biochemical analysis in Saccharomyces cerevisiae indicates that phenazine-1-carboxylic acid is harmful to vesicular trafficking and autophagy. Sci Rep 7:1967
Nakagawa H, Komori M, Nishimura K (2021) Carbon tetrachloride suppresses ER-Golgi transport by inhibiting COPII vesicle formation on the ER membrane in the RLC-16 hepatocyte cell line. Cell Biol Int 45:633–641
Verboogen DRJ, Revelo NH, Ter Beest M, van den Bogaart G (2019) Interleukin-6 secretion is limited by self-signaling in endosomes. J Mol Cell Biol 11:144–157
Acknowledgements
This research was funded by the National Natural Science Foundation of China (No. 31860010), the Natural Science Foundation of Guangxi Zhuang autonomous region (No. 2017JJA130300y and 2018GXNSFAA138008), and Science and Technology Program of Guangzhou, China (No. 201804010155).
Author information
Authors and Affiliations
Contributions
JX wrote the main part of the paper and performed the experiments. AAT, TL, DG, and WL wrote the less part of the paper. CS and ZL conceived the experiments and proofread the paper. All the authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
The authors have obtained the appropriate permission from the responsible authority for performing cell assays.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiao, J., Thwe, A.A., Liu, T. et al. Anti-Inflammatory Effects of an Extract from Pseudomonas aeruginosa and Its Purified Product 1-Hydroxyphenazine on RAW264.7 Cells. Curr Microbiol 78, 2762–2773 (2021). https://doi.org/10.1007/s00284-021-02544-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02544-3