Abstract
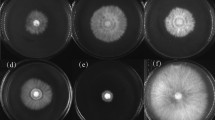
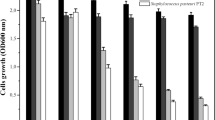
Bioprospecting sub-explored environments such as Antarctic locations leads to finding out diverse activities, reducing harmful chemical usage that affects both human health and the environment. In this study, ~ 7000 cold-adapted bacterial strains were isolated from samples around Melchior Antarctic Base at 5 °C and more than 13,000 at 15 °C. Out of them, 900 different colony morphotypes were evaluated for antimicrobial production, and 13 isolates demonstrated antibacterial and antifungal activities. One isolate, closely related to Burkholderia gladioli according to 16S rDNA (99.8%), gyrB (99.6%) and Cpn60 (99.4%) gene sequence analysis, showed a consistent, broad antimicrobial spectrum against both pathogenic and phytopathogenic bacteria. Its potent antifungal activity inhibits the growth of various plant pathogenic fungi, whereas it was mainly studied against Penicillium digitatum and Macrophomina phaseolina, the causal agents of blue mould in postharvest fruits and charcoal rot in soybean crops, respectively. The antibacterial compound exhibited low molecular weight (< 6000 Da), resistance to lytic enzymes and stability in a broad range of temperature and pHs. Observations of the B. gladioli MB39 antifungal effects over M. phaseolina mycelia by scanning electron microscopy showed alterations in hyphal structures, reduced hyphal extension, and severe cell morphology changes such as cytoplasmic leakage, flattened and empty mycelia. Here we report the isolation and identification of a cold-adapted B. gladioli strain. The results describe the effectiveness of the antarctic strain for bacterial and fungal phytopathogens biocontrol and its potential for crop protection plans.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Copping LG, Menn JJ (2000) Biopesticides: a review of their action, applications and efficacy. Pest Manage Sci 56:651–676. https://doi.org/10.1002/1526-4998(200008)56:8%3c651::AID-PS201%3e3.0.CO;2-U
Panda SK, Ray RC, Mishra SS, Kayitesi E (2018) Microbial processing of fruit and vegetable wastes into potential biocommodities: a review. Crit Rev Biotechnol 38:1–16. https://doi.org/10.1080/07388551.2017.1311295
Chevrette MG, Currie CR (2019) Emerging evolutionary paradigms in antibiotic discovery. J Ind Microbiol Biotechnol 46:257–271. https://doi.org/10.1007/s10295-018-2085-6
Lewis K (2015) Challenges of antibiotic discovery. Microbe Mag 10:363–369. https://doi.org/10.1128/microbe.10.363.1
Stincone P, Brandelli A (2020) Marine bacteria as source of antimicrobial compounds. Crit Rev Biotechnol 40:306–319. https://doi.org/10.1080/07388551.2019.1710457
Encheva-Malinova M, Stoyanova M, Avramova H et al (2014) Antibacterial potential of streptomycete strains from antarctic soils. Biotechnol Biotechnol Equip 28:721–727. https://doi.org/10.1080/13102818.2014.947066
Danilovich ME, Sánchez LA, Acosta F, Delgado OD (2018) Antarctic bioprospecting: in pursuit of microorganisms producing new antimicrobials and enzymes. Polar Biol 41:1417–1433. https://doi.org/10.1007/s00300-018-2295-4
Arnau GV, Danilovich ME, Sánchez LA et al (2016) Novel sources of antimicrobials from pristine and poorly explored environments. The Patagonia microbiota case. In: Olivera N, Libkind D, Donati E (eds) Biology and biotechnology of patagonian microorganisms. Springer International Publishing, Cham, pp 127–146
Lengai GMW, Muthomi JW (2018) Biopesticides and their role in sustainable agricultural production. J Biosci Med 06:7–41. https://doi.org/10.4236/jbm.2018.66002
Compant S, Nowak J, Coenye T et al (2008) Diversity and occurrence of Burkholderia spp. in the natural environment. FEMS Microbiol Rev 32:607–626. https://doi.org/10.1111/j.1574-6976.2008.00113.x
Estrada-De Los Santos P, Bustillos-Cristales R, Caballero-Mellado J (2001) Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Appl Environ Microbiol 67:2790–2798. https://doi.org/10.1128/AEM.67.6.2790-2798.2001
Kowalska B, Smolińsk U, Oskiera M (2015) Burkholderia gladioli associated with soft rot of onion bulbs in Poland. J Plant Pathol 97:37–43. https://doi.org/10.4454/JPP.V97I1.007
Moebius N, Ross C, Scherlach K et al (2012) Biosynthesis of the respiratory toxin bongkrekic acid in the pathogenic bacterium Burkholderia gladioli. Chem Biol 19:1164–1174. https://doi.org/10.1016/j.chembiol.2012.07.022
Depoorter E, Bull MJ, Peeters C et al (2016) Burkholderia: an update on taxonomy and biotechnological potential as antibiotic producers. Appl Microbiol Biotechnol 100:5215–5229. https://doi.org/10.1007/s00253-016-7520-x
Elshafie HS, Racioppi R, Bufo SA, Camele I (2017) In vitro study of biological activity of four strains of Burkholderia gladioli pv. agaricicola and identification of their bioactive metabolites using GC–MS. Saudi J Biol Sci 24:295–301. https://doi.org/10.1016/j.sjbs.2016.04.014
Elshafie HS, Camele I, Racioppi R et al (2012) In vitro antifungal activity of Burkholderia gladioli pv. agaricicola against some phytopathogenic fungi. Int J Mol Sci 13:16291–16302. https://doi.org/10.3390/ijms131216291
Esmaeel Q, Pupin M, Jacques P, Leclère V (2017) Nonribosomal peptides and polyketides of Burkholderia: new compounds potentially implicated in biocontrol and pharmaceuticals. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-9166-3
Ross C, Opel V, Scherlach K, Hertweck C (2014) Biosynthesis of antifungal and antibacterial polyketides by Burkholderia gladioli in coculture with Rhizopus microsporus. Mycoses 57:48–55. https://doi.org/10.1111/myc.12246
Scuderi G, Bonaccorsi A, Panebianco S et al (2009) Some strains of Burkholderia gladioli are potential candidates for postharvest biocontrol of fungal rots in citrus and apple fruits. J Plant Pathol 91:207–213. https://doi.org/10.4454/jpp.v91i1.645
Sánchez LA, Gómez FF, Delgado OD (2009) Cold-adapted microorganisms as a source of new antimicrobials. Extremophiles 13:111–120. https://doi.org/10.1007/s00792-008-0203-5
Fukushima M, Kakinuma K, Kawaguchi R (2002) Phylogenetic analysis of Salmonella, Shigella, and Escherichia coli strains on the basis of the gyrB gene sequence. J Clin Microbiol 40:2779–2785. https://doi.org/10.1128/JCM.40.8.2779
Hill JE, Town JR, Hemmingsen SM (2006) Improved template representation in cpn 60 polymerase chain reaction (PCR) product libraries generated from complex templates by application of a specific mixture of PCR primers. Environ Microbiol 8:741–746. https://doi.org/10.1111/j.1462-2920.2005.00944.x
Furuya N, Ura H, Iiyama K et al (2002) Specific oligonucleotide primers based on sequences of the 16S–23S rDNA spacer region for the detection of Burkholderia gladioli by PCR. J Gen Plant Pathol 68:220–224. https://doi.org/10.1007/PL00013080
Labrenz M, Collins M, Lawson P et al (1998) Antarctobacter heliothermus gen. nov., sp. nov., a budding bacterium from hypersaline and heliothermal Ekho Lake. Int J Syst Bacteriol 48:1363–1372. https://doi.org/10.1099/00207713-48-4-1363
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57:535–538. https://doi.org/10.1139/Z09-046
Zaidi S, Usmani S, Singh BR, Musarrat J (2006) Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64:991–997. https://doi.org/10.1016/j.chemosphere.2005.12.057
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Kremer RJ, Souissi T (2001) Cyanide production by rhizobacteria and potential for suppression of weed seedling growth. Curr Microbiol 43:182–186. https://doi.org/10.1007/s002840010284
Tenorio-Salgado S, Tinoco R, Vazquez-Duhalt R et al (2013) Identification of volatile compounds produced by the bacterium Burkholderia tropica that inhibit the growth of fungal pathogens. Bioengineered 4:236–243. https://doi.org/10.4161/bioe.23808
Arnau VG, Sánchez LA, Delgado OD (2015) Pseudomonas yamanorum sp. nov., a psychrotolerant bacterium isolated from a subantarctic environment. Int J Syst Evol Microbiol 65:424–431. https://doi.org/10.1099/ijs.0.065201-0
Sánchez LA, Hedström M, Delgado MA, Delgado OD (2010) Production, purification and characterization of serraticin A, a novel cold-active antimicrobial produced by Serratia proteamaculans 136. J Appl Microbiol 109:936–945. https://doi.org/10.1111/j.1365-2672.2010.04720.x
Khamna S, Yokota A, Lumyong S (2009) Actinomycetes isolated from medicinal plant rhizosphere soils: diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J Microbiol Biotechnol 25:649–655. https://doi.org/10.1007/s11274-008-9933-x
Debode J, De MK, Perneel M et al (2007) Biosurfactants are involved in the biological control of Verticillium microsclerotia by Pseudomonas spp. J Appl Microbiol 103:1184–1196. https://doi.org/10.1111/j.1365-2672.2007.03348.x
Yabuuchi E, Kosako Y, Oyaizu H et al (1992) Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. Nov Microbiol Immunol 36:1251–1275. https://doi.org/10.1111/j.1348-0421.1992.tb02129.x
O’Brien A, Sharp R, Russell NJ, Roller S (2004) Antarctic bacteria inhibit growth of food-borne microorganisms at low temperatures. FEMS Microbiol Ecol 48:157–167. https://doi.org/10.1016/j.femsec.2004.01.001
Tapia-Vázquez I, Sánchez-Cruz R, Arroyo-Domínguez M et al (2020) Isolation and characterization of psychrophilic and psychrotolerant plant-growth promoting microorganisms from a high-altitude volcano crater in Mexico. Microbiol Res 232:126394. https://doi.org/10.1016/j.micres.2019.126394
Shivaji S, Reddy GS, Aduri RP et al (2004) Bacterial diversity of a soil sample from Schirmacher Oasis, Antarctica. Cell Mol Biol 50:525–536. https://doi.org/10.1170/T542
Han SR, Yu SC, Ahn DH et al (2016) Complete genome sequence of Burkholderia sp. strain PAMC28687, a potential octopine-utilizing bacterium isolated from Antarctica lichen. J Biotechnol 226:16–17. https://doi.org/10.1016/j.jbiotec.2016.03.043
Kaltenpoth M, Flórez LV (2020) Versatile and dynamic symbioses between insects and Burkholderia bacteria. Annu Rev Entomol 65:145–170. https://doi.org/10.1146/annurev-ento-011019-025025
Dukare AS, Paul S, Nambi VE et al (2019) Exploitation of microbial antagonists for the control of postharvest diseases of fruits: a review. Crit Rev Food Sci Nutr 59:1498–1513. https://doi.org/10.1080/10408398.2017.1417235
Magalhães VC, de Barbosa LO, Andrade JP et al (2017) Burkholderia isolates from a sand dune leaf litter display biocontrol activity against the bole rot disease of Agave sisalana. Biol Control 112:41–48. https://doi.org/10.1016/j.biocontrol.2017.06.005
Nunes CA (2012) Biological control of postharvest diseases of fruit. Eur J Plant Pathol 133:181–196. https://doi.org/10.1146/annurev.phyto.40.120401.130158
Liu J-T, Lu X-L, Liu X-Y et al (2013) Bioactive natural products from the Antarctic and Arctic organisms. Mini-Rev Med Chem 13:617–626. https://doi.org/10.2174/1389557511313040013
Depoorter E, De Canck E, Coenye T, Vandamme P (2021) Burkholderia bacteria produce multiple potentially novel molecules that inhibit carbapenem-resistant Gram-negative bacterial pathogens. Antibiotics 10:147. https://doi.org/10.3390/antibiotics10020147
Cui G, Yin K, Lin N et al (2020) Burkholderia gladioli CGB10: a novel strain biocontrolling the sugarcane smut disease. Microorganisms 8:1943. https://doi.org/10.3390/microorganisms8121943
Lin Y-T, Lee C-C, Leu W-M et al (2021) Fungicidal activity of volatile organic compounds emitted by Burkholderia gladioli strain BBB-01. Molecules 26:745. https://doi.org/10.3390/molecules26030745
Damalas C, Koutroubas S (2016) Farmers’ exposure to pesticides: toxicity types and ways of prevention. Toxics 4:1. https://doi.org/10.3390/toxics4010001
Reznikov S, Vellicce GR, González V et al (2016) Evaluation of chemical and biological seed treatments to control charcoal rot of soybean. J Gen Plant Pathol 82:273–280. https://doi.org/10.1007/s10327-016-0669-4
Reznikov S, De Lisi V, Claps P et al (2019) Evaluation of the efficacy and application timing of different fungicides for management of soybean foliar diseases in northwestern Argentina. Crop Prot. https://doi.org/10.1016/j.cropro.2019.104844
Kumar R, Yadav S, Swain D, Jha G (2018) Burkholderia gladioli strain NGJ1 deploys a prophage tail-like protein for mycophagy. Microb Cell 5:116–118. https://doi.org/10.15698/mic2018.02.617
Acknowledgements
Thanks are due to Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Instituto Antártico Argentino (IAA) for allowing us to work on board of the Oceanographic Vessel ‘‘Puerto Deseado’’—CONICET and to all the crew for their help and assistance.
Funding
These findings were funded by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP0662; PIO CONICET-UNCa); Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT, PICT 2679) and Secretaría de Ciencia y Tecnología de la Universidad Nacional de Catamarca (SECyT UNCa, 02/L442).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work and the manuscript was critically reviewed by all of the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
The authors declare that ethical standards have been followed and no human participants or animals were involved in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sarli, D.A., Sánchez, L.A. & Delgado, O.D. Burkholderia gladioli MB39 an Antarctic Strain as a Biocontrol Agent. Curr Microbiol 78, 2332–2344 (2021). https://doi.org/10.1007/s00284-021-02492-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02492-y