Abstract
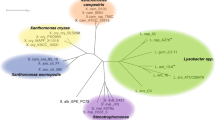
Lactococcus lactis subsp. lactis KF147 as a non-dairy strain from lactic acid bacteria (LAB) can inhabit plant tissues. It can grow on complex carbohydrates derived from plant cell walls. Its genome size is one of the largest among the sequenced lactococcal strains, possessing many genes that do not have homologues in the published genome sequences of dairy-associated L. lactis strains. In silico analysis has identified a gene cluster encoding a hybrid NRPS-PKS system (composed of non-ribosomal peptide synthetases and polyketide synthases) in the L. lactis KF147 genome, as first example of a LAB possessing such hybrid mega-enzymes. Hybrid systems produce hybrid NRP-PK secondary metabolites (natural products) in a wide variety of bacteria, fungi, and plants. In the hybrid NRPS-PKS system of L. lactis KF147, a total of 21 NRPS and 8 PKS domains were identified that are arranged into 6 NRPS modules, 3 PKS modules, and two single functional domains (trans-acyl-transferase “transAT” and thioesterase). We found homologous hybrid systems having similar gene, module, and domain organization in six other L. lactis strains and 25 strains of the dental cariogenic Streptococcus mutans. This study mainly aimed to predict the structure and function of the hybrid NRP-PK product of L. lactis KF147 using comparative genomics techniques, and included a detailed analysis of the regulatory system. Various bioinformatical approaches were used to predict the substrate specificity of the six A domains and the iterative transAT domain. Functional conservation of the A domains within different-niche-associated strains supported the prediction of the primary core structure of the putative hybrid natural product to be Leu-DLeu-Asp-DAsn-Gly-MC-MC-MC-DAsp (MC = Malonyl-CoA). Oxidative stress resistance and biofilm formation are the most probable functions of this hybrid system. The need for such a system in two different niches is argued, as an adaptation of L. lactis and S. mutans to adhere to plant tissues and human teeth, respectively, in an oxidative environment.




Similar content being viewed by others
References
Siezen RJ, Bayjanov J, Renckens B, Wels M, van Hijum SA, Molenaar D, van Hylckama Vlieg JE (2010) Complete genome sequence of Lactococcus lactis subsp. lactis KF147, a plant-associated lactic acid bacterium. J Bacteriol 192(10):2649–2650. https://doi.org/10.1128/JB.00276-10
Siezen RJ, Starrenburg MJ, Boekhorst J, Renckens B, Molenaar D, van Hylckama Vlieg JE (2008) Genome-scale genotype-phenotype matching of two Lactococcus lactis isolates from plants identifies mechanisms of adaptation to the plant niche. Appl Environ Microbiol 74(2):424–436. https://doi.org/10.1128/AEM.01850-07
Kelleher P, Bottacini F, Mahony J, Kilcawley KN, van Sinderen D (2017) Comparative and functional genomics of the Lactococcus lactis taxon; insights into evolution and niche adaptation. BMC Genomics 18(1):267. https://doi.org/10.1186/s12864-017-3650-5
Vaughan EE, Pridmore RD, Mollet B (1998) Transcriptional regulation and evolution of lactose genes in the galactose-lactose operon of Lactococcus lactis NCDO2054. J Bacteriol 180(18):4893–4902
Wegmann U, O'Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, Ventura M, Goesmann A, Gasson MJ, Kuipers OP, van Sinderen D, Kok J (2007) Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol 189(8):3256–3270. https://doi.org/10.1128/JB.01768-06
Donadio S, Monciardini P, Sosio M (2007) Polyketide synthases and nonribosomal peptide synthetases: the emerging view from bacterial genomics. Nat Prod Rep 24(5):1073–1109. https://doi.org/10.1039/b514050c
Miyanaga A, Kudo F, Eguchi T (2018) Protein-protein interactions in polyketide synthase-nonribosomal peptide synthetase hybrid assembly lines. Nat Prod Rep 35(11):1185–1209. https://doi.org/10.1039/c8np00022k
Gallo A, Ferrara M, Perrone G (2013) Phylogenetic study of polyketide synthases and nonribosomal peptide synthetases involved in the biosynthesis of mycotoxins. Toxins 5(4):717–742. https://doi.org/10.3390/toxins5040717
Singh M, Chaudhary S, Sareen D (2017) Non-ribosomal peptide synthetases: identifying the cryptic gene clusters and decoding the natural product. J Biosci 42(1):175–187
Wang H, Fewer DP, Holm L, Rouhiainen L, Sivonen K (2014) Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes. Proc Natl Acad Sci USA 111(25):9259–9264. https://doi.org/10.1073/pnas.1401734111
Martínez-Núñez MA, López VEL (2016) Nonribosomal peptides synthetases and their applications in industry. Sustain Chem Process 4:13
Siezen RJ, Khayatt BI (2008) Natural products genomics. Microb. Biotechnol 1(4):275–282. https://doi.org/10.1111/j.1751-7915.2008.00044.x
Sussmuth RD, Mainz A (2017) Nonribosomal peptide synthesis-principles and prospects. Angew Chem 56(14):3770–3821. https://doi.org/10.1002/anie.201609079
Winn M, Fyans JK, Zhuo Y, Micklefield J (2016) Recent advances in engineering nonribosomal peptide assembly lines. Nat Prod Rep 33(2):317–347. https://doi.org/10.1039/c5np00099h
Du L, Sanchez C, Shen B (2001) Hybrid peptide-polyketide natural products: biosynthesis and prospects toward engineering novel molecules. Metab Eng 3(1):78–95. https://doi.org/10.1006/mben.2000.0171
Alvarez-Sieiro P, Montalban-Lopez M, Mu D, Kuipers OP (2016) Bacteriocins of lactic acid bacteria: extending the family. Appl Microbiol Biotechnol 100(7):2939–2951. https://doi.org/10.1007/s00253-016-7343-9
Gao Y, Lu Y, Teng KL, Chen ML, Zheng HJ, Zhu YQ, Zhong J (2011) Complete genome sequence of Lactococcus lactis subsp. lactis CV56, a probiotic strain isolated from the vagina of healthy women. J Bacteriol. https://doi.org/10.1128/JB.00358-11
Mokoena MP (2017) Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules. https://doi.org/10.3390/molecules22081255
Perez RH, Zendo T, Sonomoto K (2014) Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb Cell Fact 13(Suppl 1):S3. https://doi.org/10.1186/1475-2859-13-S1-S3
Silva CCG, Silva SPM, Ribeiro SC (2018) Application of bacteriocins and protective cultures in dairy food preservation. Frontiers in Microbiology 9:594. https://doi.org/10.3389/fmicb.2018.00594
Todorov SD, Botes M, Danova ST, Dicks LM (2007) Probiotic properties of Lactococcus lactis ssp. lactis HV219, isolated from human vaginal secretions. J Appl Microbiol 103(3):629–639. https://doi.org/10.1111/j.1365-2672.2007.03290.x
Woraprayote W, Malila Y, Sorapukdee S, Swetwiwathana A, Benjakul S, Visessanguan W (2016) Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci 120:118–132. https://doi.org/10.1016/j.meatsci.2016.04.004
Khayatt BI, Siezen RJ (2008) Non-ribosomal peptide synthetases (NRPSs) and Polyketide synthases (PKSs) encoded in lactic acid bacteria. In: 9th Symposium on lactic acid bacteria, Egmond aan Zee, The Netherlands, 2008. Federation of Europesn Microbiological Societies and the Netherlands Society for Microbiology
Siezen RJ, Bayjanov JR, Felis GE, van der Sijde MR, Starrenburg M, Molenaar D, Wels M, van Hijum SA, van Hylckama Vlieg JE (2011) Genome-scale diversity and niche adaptation analysis of Lactococcus lactis by comparative genome hybridization using multi-strain arrays. Microb Biotechnol 4(3):383–402. https://doi.org/10.1111/j.1751-7915.2011.00247.x
Wu C, Cichewicz R, Li Y, Liu J, Roe B, Ferretti J, Merritt J, Qi F (2010) Genomic island TnSmu2 of Streptococcus mutans harbors a nonribosomal peptide synthetase-polyketide synthase gene cluster responsible for the biosynthesis of pigments involved in oxygen and H2O2 tolerance. Appl Environ Microbiol 76(17):5815–5826. https://doi.org/10.1128/AEM.03079-09
Golomb BL, Yu AO, Coates LC, Marco ML (2018) The Lactococcus lactis KF147 nonribosomal peptide synthetase/polyketide synthase system confers resistance to oxidative stress during growth on plant leaf tissue lysate. MicrobiologyOpen. https://doi.org/10.1002/mbo3.531
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389–3402
Blin K, Wolf T, Chevrette MG, Lu X, Schwalen CJ, Kautsar SA, Suarez Duran HG, de Los Santos ELC, Kim HU, Nave M, Dickschat JS, Mitchell DA, Shelest E, Breitling R, Takano E, Lee SY, Weber T, Medema MH (2017) antiSMASH 4.0-improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res 45:W36–W41. https://doi.org/10.1093/nar/gkx319
Khater S, Gupta M, Agrawal P, Sain N, Prava J, Gupta P, Grover M, Kumar N, Mohanty D (2017) SBSPKSv2: structure-based sequence analysis of polyketide synthases and non-ribosomal peptide synthetases. Nucleic Acids Res 45:W72–W79. https://doi.org/10.1093/nar/gkx344
Tae H, Kong EB, Park K (2007) ASMPKS: an analysis system for modular polyketide synthases. BMC Bioinform 8:327. https://doi.org/10.1186/1471-2105-8-327
Rottig M, Medema MH, Blin K, Weber T, Rausch C, Kohlbacher O (2011) NRPSpredictor2—a web server for predicting NRPS adenylation domain specificity. Nucleic Acids Res 39:W362–W367. https://doi.org/10.1093/nar/gkr323
Khayatt BI, Overmars L, Siezen RJ, Francke C (2013) Classification of the adenylation and acyl-transferase activity of NRPS and PKS systems using ensembles of substrate specific hidden Markov models. PLoS ONE 8(4):e62136. https://doi.org/10.1371/journal.pone.0062136
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59(3):307–321. https://doi.org/10.1093/sysbio/syq010
Conti E, Stachelhaus T, Marahiel MA, Brick P (1997) Structural basis for the activation of phenylalanine in the non-ribosomal biosynthesis of gramicidin S. EMBO J 16(14):4174–4183
Serre L, Verbree EC, Dauter Z, Stuitje AR, Derewenda ZS (1995) The Escherichia coli malonyl-CoA:acyl carrier protein transacylase at 1.5-A resolution. Crystal structure of a fatty acid synthase component. J Biol Chem 270(22):12961–12964
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32(5):1792–1797. https://doi.org/10.1093/nar/gkh34032/5/1792
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948. https://doi.org/10.1093/bioinformatics/btm404
Huson DH, Scornavacca C (2012) Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Syst Biol 61(6):1061–1067. https://doi.org/10.1093/sysbio/sys062
Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2013) GenBank. Nucleic Acids Res 41:D36–D42. https://doi.org/10.1093/nar/gks1195
UniProt C (2012) Reorganizing the protein space at the Universal Protein Resource (UniProt). Nucleic Acids Res 40:D71–D75. https://doi.org/10.1093/nar/gkr981
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882
Letunic I, Doerks T, Bork P (2015) SMART: recent updates, new developments and status in 2015. Nucleic Acids Res 43:D257–D260. https://doi.org/10.1093/nar/gku949
Stachelhaus T, Mootz HD, Marahiel MA (1999) The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem Biol 6(8):493–505. https://doi.org/10.1016/S1074-5521(99)80082-9
Cheng YQ, Tang GL, Shen B (2002) Identification and localization of the gene cluster encoding biosynthesis of the antitumor macrolactam leinamycin in Streptomyces atroolivaceus S-140. J Bacteriol 184(24):7013–7024
Chen XH, Vater J, Piel J, Franke P, Scholz R, Schneider K, Koumoutsi A, Hitzeroth G, Grammel N, Strittmatter AW, Gottschalk G, Sussmuth RD, Borriss R (2006) Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB 42. J Bacteriol 188(11):4024–4036. https://doi.org/10.1128/JB.00052-06
Perlova O, Gerth K, Kaiser O, Hans A, Muller R (2006) Identification and analysis of the chivosazol biosynthetic gene cluster from the myxobacterial model strain Sorangium cellulosum So ce56. J Biotechnol 121(2):174–191. https://doi.org/10.1016/j.jbiotec.2005.10.011
Tatsuno S, Arakawa K, Kinashi H (2007) Analysis of modular-iterative mixed biosynthesis of lankacidin by heterologous expression and gene fusion. J Antibiot 60(11):700–708. https://doi.org/10.1038/ja.2007.90
Tu Q, Herrmann J, Hu S, Raju R, Bian X, Zhang Y, Muller R (2016) Genetic engineering and heterologous expression of the disorazol biosynthetic gene cluster via Red/ET recombineering. Sci Rep 6:21066. https://doi.org/10.1038/srep21066
Acknowledgements
We would like to thank Professor Rene De Mot and Professor Rob Lavigne for their valuable suggestions and comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
284_2019_1799_MOESM2_ESM.pdf
L. lactis and S. mutans strains coding for the hybrid NRPS-PKS systems homologous to the hybrid NRPS-PKS system of L. lactis KF147 (PDF 34 kb)
284_2019_1799_MOESM6_ESM.pdf
Four parts of a bootstrap phylogenetic tree, showing clustering of the NRPS A domains (from A1 to A6) of L. lactis KF147 (PDF 373 kb)
284_2019_1799_MOESM12_ESM.pdf
Multiple sequence alignment of the output (DNA-binding) domain of the response regulators of the L. lactis and S. mutans strains. And multiple sequence alignment of the upstream regions of the highly conserved genes downstream of the TC systems of L. lactis and S. mutans strains (PDF 515 kb)
Rights and permissions
About this article
Cite this article
Khayatt, B.I., van Noort, V. & Siezen, R.J. The Genome of the Plant-Associated Lactic Acid Bacterium Lactococcus lactis KF147 Harbors a Hybrid NRPS-PKS System Conserved in Strains of the Dental Cariogenic Streptococcus mutans. Curr Microbiol 77, 136–145 (2020). https://doi.org/10.1007/s00284-019-01799-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-019-01799-1