Abstract
Sixty-three clinical isolates of Acinetobacter calcoaceticus–baumannii complex were analyzed for the presence of integrons and antimicrobial resistance. Class 1 integrons were detected in 40 (63.5 %) isolates. None of them had class 2 or class 3 integrons. The majority of the integrons contained aacC1–orfA–orfB–aadA1 gene cassette array. The presence of integrons was associated with the increased frequency of resistance to 12 of 15 antimicrobials tested, multi-drug resistance phenotype, and the overall resistance ranges of the strains.
Similar content being viewed by others
Introduction
Acinetobacter spp. are Gram-negative, strictly aerobic bacteria ubiquitous in the environment and in clinical settings. The genus comprises 33 named and unnamed genospecies. The Acinetobacter calcoaceticus–baumannii complex includes four genospecies, i.e., A. calcoaceticus, A. baumannii, and hybridization groups 3 and 13 TU that cannot be differentiated by phenotypic tests [8]. Members of the A. calcoaceticus–baumannii complex account for about 75 % of Acinetobacter spp. isolated from clinical specimens [14] and are etiological agents of a wide spectrum of nosocomial infections, including blood and urinary tract infections, and pneumonia [22, 34]. Many infections can be severe with mortality ranging from 26 to 70 % [28].
Antimicrobial resistance of A. calcoaceticus–baumannii complex isolates has been increasing continuously [21]. Clinical isolates are frequently resistant to most antimicrobials commonly used against Gram-negative pathogens. In recent years, a growing number of carbapenem-resistant strains have been reported in Europe [13].
One of the genetic elements involved in the spread of resistance among bacteria are integrons. They are genetic elements responsible for integration and rearrangements of resistance determinants called gene cassettes [32]. Integrons consist of an integrase gene, a primary recombination site called attI, and a promoter PC that directs transcription of the integrated genes. Several classes of integrons are distinguished upon the sequences of the integrase genes. The most common integrons belong to class 1 and play an important role in the emergence and spread of resistance genes [2, 31]. Bacterial strains harboring class 1 integrons are usually multiresistant, i.e., resistant to antimicrobials of at least three different classes. This has been proven for many species of the Enterobacteriaceae family, including Escherichia coli, Enterobacter hormaechei, E. aerogenes, Citrobacter freundii, Klebsiella pneumoniae, K. oxytoca, Serratia marcescens, and Proteus mirabilis [17, 24, 25].
The aim of this study was to investigate the presence of integrons in clinical isolates of the A. calcoaceticus–baumannii complex, with a focus on antimicrobial resistance of the isolates and gene cassette content of the integrons.
Materials and Methods
Bacterial Isolates
Sixty-three A. calcoaceticus–baumannii complex isolates were cultured from specimens taken from inpatients at a hospital in Poznań, Poland, and identified with API 20NE (bioMérieux). The isolates were collected between 2000 and 2010. The specimens included urine, blood, wound swabs, and tracheobronchial aspirates.
BOX–PCR Typing
The genetic similarity of the isolates was determined by BOX–PCR typing with BOX A1R primer according to Versalovic et al. [37]. The PCR products were separated in agarose gel, stained with ethidium bromide and digitalized with Bio-Print v. 99 gel documentation system (Vilbert-Lourmat). The electrophoretic patterns were analyzed by GelCompar II 3.5 software (Applied Maths) with internal and external normalization. Optimization and band position tolerance were set at 1 %. Similarity between fingerprints was calculated with the Dice coefficient. Clustering was carried out by the unweighted pair–group method with average linkages (UPGMA).
Detection of Integrase Genes
Multiplex PCR assay for the detection of intI1, intI2, and intI3 integrase genes characteristics for class 1, class 2, and class 3 integrons, respectively, was performed according to Dillon et al. [4]. Amplification involved an initial denaturation (94 °C, 5 min) followed by 30 cycles of denaturation (94 °C, 1 min), annealing (59 °C, 1 min), and extension (72 °C, 1 min) with a final extension step (72 °C, 5 min).
Analysis of the Variable Regions of Class 1 Integrons
The variable regions of class 1 integrons were PCR-amplified with primers 5′-CS and 3′-CS recommended by Lévesque et al. [18]. The PCR reaction was conducted as follows: initial denaturation 94 °C, 5 min, and 30 cycles of 94 °C 1 min., 55 °C 1 min., 72 °C 5 min., and final elongation 72 °C 8 min. The amplicons were purified with ExoSAP-IT (Affymetrix) and sequenced in a 3130xl Genetic Analyzer (Applied Biosystems). The sequences were assembled with DNA Baser (HeracleSoftware) and aligned with available GenBank data using nucleotide BLAST (Basic Local Alignment Search Tool).
All PCR reactions were carried out in a C1000 Thermal Cycler (BioRad) with primers synthesized by Oligo.pl and HiFi Taq polymerase provided by Novazym. The PCR products were separated in 1.5 % agarose gel (Novazym). Molecular weight of PCR products was determined with GelCompar II 3.5. The experiments were performed in duplicate.
Detection of Integrons in Plasmids
Plasmid DNA was isolated using Plasmid Mini AX (A&A Biotechnology) kit following manufacturer’s instruction. Plasmids were separated in a 0.7 % agarose gel, and molecular size of the bands was determined with GelCompar II 3.5; E. coli V-517 plasmids were used as molecular size markers. All plasmids were cut off the gel and eluted by Gel-Out kit (A&A Biotechnology). Integrons in plasmids were detected as described above.
Antibiotic Susceptibility Testing
Antibiotic susceptibility of the isolates was determined by standard disk diffusion method on Mueller-Hinton agar (Oxoid) according to the guidelines and breakpoints of Clinical and Laboratory Standards Institute [3]. The isolates were tested for susceptibility to 15 antibiotics representing seven classes: amikacin (30 µg), ampicillin/sulbactam (20 µg), cefepime (30 µg) cefotaxime (30 µg), ceftazidime (30 µg), chloramphenicol (30 µg), ciprofloxacine (5 µg), gentamicin (10 µg), imipenem (10 µg), piperacillin (100 µg), piperacillin/tazobactam (110 µg), sulfamethoxazole/trimethoprim (co-trimoxazole) (25 µg), tetracycline (30 µg), and tobramycin (10 µg). Resistance to netilmicin (10 µg) was determined according to European Committee on Antimicrobial Susceptibility Testing breakpoints v. 3.0 [5] All antibiotic disks were provided by Oxoid. The quality control strain was E. coli ATCC 25922.
Statistical Analysis
The frequencies of resistance to particular antimicrobials in integron-positive and -negative isolates were compared with Fisher’s exact test. The differences in antimicrobial resistance ranges, expressed as the number of antimicrobial classes to which the isolates were resistant, were compared with Mann–Whitney U test. P < 0.05 was considered to indicate statistical significance. Calculations were performed with Statistica 10 software (StatSoft).
Results
We analyzed 63 clinical isolates of the A. calcoaceticus–baumannii complex for the presence of integrons and their antimicrobial resistance. In order to avoid analyzing repetitive clones, we carried out BOX–PCR typing. Although it indicated the presence of two clusters consisting of three and two isolates with identical band patterns (Supplementary Fig. S1), subsequent analyses showed differences between their resistance profiles, which may be explained by localization of some antimicrobial resistance determinants on plasmids.
Screening for the presence of integron integrase genes showed the presence of class 1 integrase gene in 40 (63.5 %) clinical isolates of A. calcoaceticus–baumannii complex. None of the isolates had class 2 or class 3 integrons. Class 1 integron-harboring strains carried several plasmids (Supplementary Fig. S2); however, PCR analysis of the plasmids showed the integrons were localized only in a ~60-kbp plasmid.
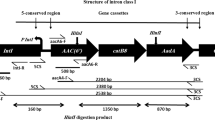
The amplification of the variable regions of class 1 integrons yielded a 2.5-kbp amplicon in 34 isolates and a 1.9-kbp PCR product in one isolate. We did not obtain an amplicon of the variable region in five (12.5 %) integron-positive isolates. Sequence analysis showed the presence of gene cassettes conferring resistance to aminoglycosides (aadA1, aadA2, and aacC1), trimethoprim (dfrA12), as well as genes of unknown function. All of the 2.5-kbp amplicons consisted of aacC1–orfA–orfB–aadA1 gene cassette arrays, whereas the 1.9-kbp amplicon contained a dfrA12–orfF–aadA2 array.
The frequencies of antimicrobial resistance of integron-positive and integron-negative A. calcoaceticus–baumannii complex isolates are presented in Table 1. Among the integron-positive strains, the highest resistance frequency was noted for cefepime, cefotaxime, and tetracycline, and the lowest to netilmicin. Strains without integrons were also most frequently resistant to cefepime and least frequently resistant to netilmicin. For most antimicrobials, the frequency of resistance was higher in the group of strains that harbored class 1 integrons. No statistically significant differences were found for imipenem, netilmicin, and tobramycin.
The integron-bearing strains were resistant to 3–14 of the antimicrobials belonging to 3–7 classes, i.e., all of them were multi-drug resistant (Fig. 1). The integron-negative isolates were resistant to 0–14 drugs of 0–7 classes and fifteen of them (65.2 %) were multi-drug resistant (MDR). The difference in the MDR frequency between strains with and without integrons was statistically significant (P < 0.001, Fisher’s exact test). The differences in resistance ranges, defined either as the number of antimicrobials or antimicrobial classes the strains were resistant to, between integron-positive and -negative strains were statistically significant (P < 0.001, U Mann–Whitney test).
Discussion
Acinetobacter calcoaceticus–baumannii complex has become an emerging etiological agent of nosocomial infections [11]. Surveys have shown increasing resistance among clinical isolates and emergence of MDR strains, likely due to overuse of antimicrobials, which limits therapeutic options. One of the most effective drugs against A. calcoaceticus–baumannii complex are carbapenems. In England, approximately 15 % of isolates were resistant to imipenem [35]; however, in Spain, higher frequency of resistance to imipenem has been reported, ranging from 21 to 80 %, depending on location and the time of study [36]. In Asia, resistance to imipenem among A. calcoaceticus–baumannii complex has been also reported, with frequency reaching 36 % in Nepal [23] and 59 % in Taiwan [15]. In our study, 41 % of isolates were resistant to imipenem, whereas Bogiel et al. [1] have reported 27.6–31.0 % of imipenem-resistant strains isolated from a hospital in Bydgoszcz, Poland, and Wróblewska et al. [38] have found 13.6 % of isolates intermediately susceptible or resistant to that drug in a hospital in Warsaw.
Genes determining antimicrobial resistance in Gram-negative bacteria are often located within integrons [2]. We found class 1 integrons in 63.5 % of A. calcoaceticus–baumannii complex isolates, which is similar to the frequency of integron-positive strains among A. baumannii isolated in Iran—74.0 % [28], Taiwan—71.4 % [9], and South Africa—65.6 % [30]. Much higher percentage (80.4 %) was observed among A. calcoaceticus–baumannii complex strains originated from a hospital in China [19]. Class 1 integrons in Acinetobacter spp. have been localized in different plasmids, ranging from 60 kbp [12, this study] to 120 kbp [20], but they can occur in the chromosome as well [20]. We did not detect the presence of class 2 and class 3 integrons. Class 2 integrons are found with low frequency in various Gram-negative bacteria [25, 26, 39]. In Acinetobacter spp., class 2 integron has been found only in limited number of strains, originated from a hospital in Brazil [7], from hospitals in Iran [10, 29, 33], and from wastewater in China [39]. Class 3 integrons have not been detected in Acinetobacter spp. so far.
There was little diversity in the genetic content of the class 1 integrons. We identified only two gene cassette arrays: aacC1–orfA–orfB–aadA1 and dfrA12–orfF–aadA2, conferring resistance to aminoglycosides and trimethoprim, and all of the integron-positive isolates displayed resistance to those antimicrobials. As much as 12.5 % of the isolates did not yield a PCR product of the integron’s variable region, which can be explained by altered sequence or the lack of sul1 gene in the 3′ conserved region of class 1 integrons [16]. The same gene cassette arrays have been found in A. baumannii in Taiwan [9]. An A. calcoaceticus–baumannii complex strain isolated from Poland contained a plasmid-located integron with bla VIM-2- aacA4 gene array [6].
The genes of class 1 integrons of A. calcoaceticus–baumannii complex coded only for resistance to aminoglycosides and trimethoprim. Nevertheless, resistance to penicillins, cephalosporins, ciprofloxacin, tetracycline, and sulfamethoxazole/trimethoprim was associated with the presence of an integron as well, and all strains with integrons were multi-drug resistant. This phenomenon appears to be common in integron-bearing bacteria [10, 24, 25], likely to the presence of numerous resistance determinants in one genetic element, e.g., a plasmid. This may also explain higher resistance ranges of integron-positive A. calcoaceticus–baumannii complex strains in this study, as the integrons were located in a 60-kbp plasmid. It has been proved that multidrug resistance phenotype in bacteria, especially those of nosocomial origin, can be spread through plasmids containing class 1 integrons that can be readily transferred between different species [27].
In conclusion, the presence of class 1 integrons among clinical isolates of A. calcoaceticus–baumannii complex appears to be common in Poland. Since they are associated with MDR phenotype, the presence of class 1 integrons may be used as a marker of multi-drug resistance.
References
Bogiel T, Kwiecińska-Piróg J, Jachna-Sawicka K, Gospodarek E (2010) Carbapenem-resistant Acinetobacter baumannii strains [in Polish]. Med Dosw Mikrobiol 62:127–134
Cambray G, Guerout AM, Mazel D (2010) Integrons. Annu Rev Genet 44:141–166
CLSI (2009) Performance standards for antimicrobial disk susceptibility tests; approved standard, 10th edn. CLSI document M02–A10, vol. 29, p 1
Dillon B, Thomas L, Mohmand G, Zelynsky A, Iredell J (2005) Multiplex PCR for screening of integrons in bacterial lysates. J Microbiol Methods 62:221–232
EUCAST (2013) Breakpoint tables for interpretation of MICs and zone diameters. Version 3.0
Fiett J, Baraniak A, Mrówka A, Kleischer M, Drulis-Kawa Z, Naumiuk Ł, Samet A, Hryniewicz W, Gniadkowski M (2006) Molecular epidemiology of acquired-metallo-β-lactamase-producing bacteria in Poland. Antimicrob Agents Chemother 50:880–886
Fonseca ÉL, Freitas FDS, Scheidegger EMD, Jacinto T, Vicente ACP (2011) Class 2 integrons in multidrug-resistant Acinetobacter baumannii circulating in different Brazilian geographic regions. Int J Antimicrob Agents 38:95–96
Gerner-Smidt P, Tjernberg I, Ursing J (1991) Reliability of phenotypic tests for identification of Acinetobacter species. J Clin Microbiol 29:277–282
Huang LY, Chen TL, Lu PL, Tsai CA, Cho WL, Chang FY, Fung CP, Siu LK (2008) Dissemination of multidrug-resistant, class 1 integron-carrying Acinetobacter baumannii isolates in Taiwan. Clin Microbiol Infect 14:1010–1019
Japoni S, Japoni A, Farshad S, Ali AA, Jamalidoust M (2011) Association between existence of integrons and multi-drug resistance in Acinetobacter isolated from patients in Southern Iran. Pol J Microbiol 60:163–168
Joly-Guillou ML (2005) Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect 11:868–873
Kaase M, Szabados F, Pfennigwerth N, Anders A, Geis G, Pranada A, Röbler S, Lang U, Gatermann S (2014) Description of the metallo-β-lactamase GIM-1 in Acinetobacter pittii. J Antimicrob Chemother 69:81–84
Kempf M, Rolain JM (2012) Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents 39:105–114
Krzymińska S, Frąckowiak H, Kaznowski A (2012) Acinetobacter calcoaceticus–baumannii complex strains induce caspase-dependent and caspase-independent death of human epithelial cells. Curr Microbiol 65:319–329
Kuo SC, Chang SC, Wang HY, Lai JF, Chen PC, Shiau YR, Huang IW, Lauderdfale TLY (2012) Emergence of extensively drug-resistant Acinetobacter baumannii complex over 10 years: Nationwide data from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program. BMC Infect Dis 12:200
Laroche E, Pawlak B, Berthe T, Skurnik D, Petit F (2009) Occurrence of antibiotic resistance and class 1, 2 and 3 integrons in Escherichia coli isolated from a densely populated estuary (Seine, France). FEMS Microbiol Ecol 68:118–130
Leverstein–van Hall MA, Blok HEM, Rogier A, Donders T, Paauw A, Fluit AC, Verhoef J (2003) Multidrug resistance among Enterobacteriaceae is strongly associated with the presence of integrons and is independent of species or isolate origin. J Infect Dis 187:251–259
Lévesque C, Piché L, Larose C, Roy PH (1995) PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother 39:85–191
Lin L, Ling BD, Li XZ (2009) Distribution of the multidrug efflux pump genes, adeABC, adeDE and adeIJK, and class 1 integron genes in multiple-antimicrobial-resistant clinical isolates of Acinetobacter baumannii–Acinetobacter calcoaceticus complex. Int J Antimicrob Agents 33:27–32
Liu SY, Lin JY, Chu C, Su LH, Lin TY, Chiu CH (2006) Integron-associated imipenem resistance in Acinetobacter baumannii isolated from a regional hospital in Taiwan. Int J Antimicrob Agents 27:81–84
Maragakis LL, Perl TM (2008) Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 46:1254–1263
McConnell MJ, Actis L, Pachón J (2013) Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev 37:130–155
Mishra SK, Rijal BP, Pokhrel BM (2013) Emerging threat of multidrug resistant bugs—Acinetobacter calcoaceticus–baumannii complex and methicillin resistant Staphylococcus aureus. BMC Res Notes 6:95
Mokracka J, Koczura R, Pawłowski K, Kaznowski A (2011) Resistance patterns and integron cassette arrays of Enterobacter cloacae complex strains of human origin. J Med Microbiol 60:737–743
Mokracka J, Gruszczyńska B, Kaznowski A (2012) Integrons, β-lactamase and qnr genes in multidrug resistant clinical isolates of Proteus mirabilis and P. vulgaris. APMIS 120:950–958
Mokracka J, Koczura R, Kaznowski A (2012) Multiresistant Enterobacteriaceae with class 1 and class 2 integrons in a municipal wastewater treatment plant. Water Res 46:3353–3363
Mooij JM, Willemsen I, Lobbrecht M, Vandebroucke-Grauls C, Kluytmans J, Savelkoul PHM (2009) Integron class 1 reservoir among highly resistant Gram-negative microorganisms recovered at a Dutch teaching hospital. Infect Control Hosp Epidemiol 30:1015–1018
Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582
Peymani A, Farajnia S, Nahei MR, Sohrabi N, Abbasi L, Ansarin K, Azhari F (2012) Prevalence of class 1 integron among multidrug-resistant Acinetobacter baumannii in Tabriz, Northwest of Iran. Pol J Microbiol 61:57–60
Segal H, Thomas R, Elisha BG (2003) Characterization of class 1 integron resistance gene cassettes and the identification of a novel IS-like element in Acinetobacter baumannii. Plasmid 49:169–178
Stokes HW, Gillings MR (2011) Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS Microbiol Rev 35:790–819
Stokes HW, Hall RM (1989) A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol 3:1669–1683
Taherikalani M, Maleki A, Sadeghifard N, Mohammadzadeh D, Soroush S, Asadollahi P, Asadollahi K, Emaneini M (2011) Dissemination of class 1, 2 and 3 integrons among different multidrug resistant isolates of Acinetobacter baumannii in Tehran hospitals, Iran. Pol J Microbiol 60:169–174
Towner KJ (1997) Clinical importance and antibiotic resistance of Acinetobacter spp. J Med Microbiol 46:721–746
Towner KJ (2008) Acinetobacter: an old friend, but a new enemy. J Hosp Infect 73:355–363
Van Looveren M, Goossens H, the ARPAC Steering Group (2004) Antimicrobial resistance of Acinetobacter spp. in Europe. Clin Microbiol Infect 10:684–704
Versalovic J, Schneider M, de Bruin FJ, Lupski JR (1991) Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol 5:25–40
Wróblewska MM, Towner KJ, Marchel H, Łuczak M (2007) Emergence and spread of carbapenem-resistant strains of Acinetobacter baumannii in a tertiary-care hospital in Poland. Clin Microbiol Infect 13:490–496
Xia R, Ren Y, Guo X, Xu H (2013) Molecular diversity of class 2 integrons in antibiotic-resistant Gram-negative bacteria found in wastewater environments in China. Ecotoxicology 22:402–414
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
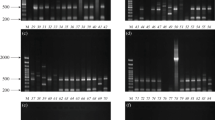
Dendrogram showing antimicrobial resistance profiles and genetic relatedness of 63 A. calcoaceticus–baumannii complex isolates determined by BOX–PCR analysis with Dice coefficient and UPGMA clustering method. Antimicrobial symbols: AMK amikacin, GEN gentamicin, NET netilmicin, TOB tobramycin, SAM ampicillin/sulbactam, PIP piperacillin, TZP piperacillin/tazobactam, TIC ticarcillin, FEP cefepime, CTX cefotaxime, CAZ ceftazidime, IMP imipenem, CIP ciprofloxacin, TET tetracycline, SXT sulfamethoxazole/trimethoprim (PDF 333 kb)
Supplementary Fig. S2
Agarose gel electrophoresis of plasmids extracted from class 1 integron-bearing A. calcoaceticus–baumannii complex isolates. Line 1, E. coli V-517 (plasmid sizes in kpb given on the left); lines 2–9, A. calcoaceticus–baumannii complex isolates. Plasmids containing class 1 integrons indicated by white arrow (PDF 254 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Koczura, R., Przyszlakowska, B., Mokracka, J. et al. Class 1 Integrons and Antibiotic Resistance of Clinical Acinetobacter calcoaceticus–baumannii Complex in Poznań, Poland. Curr Microbiol 69, 258–262 (2014). https://doi.org/10.1007/s00284-014-0581-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0581-0