Abstract
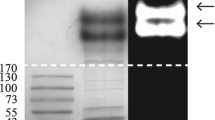
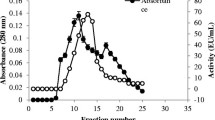
In the present work, Aspergillus fumigatus is described as a higher producer of hydrolytic enzymes secreted in response to the presence of the Callosobruchus maculatus bruchid pest. This fungus was able to grow over cowpea weevil shells as a unique carbon source, secreting alkaline proteolytic and chitinolytic enzymes. Enzyme secretion in A. fumigatus was induced by both C. maculatus exoskeleton as well as commercial chitin, and alkaline proteolytic and chitinolytic activities were detected after 48 hours of growth. Furthermore, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis showed the production of specific proteins. Among them, two extracellular alkaline proteinases from culture enriched with C. maculatus exoskeleton were purified after chromatographic procedures using ion exchange and affinity columns. These proteins, named AP15 and AP30, had apparent molecular masses of 15,500 and 30,000 Da, respectively, as estimated by SDS-PAGE electrophoresis and mass spectrometry. AP30 was classified as a serine proteinase because it was inhibited by 5 mM phenylmethylsulfonyl fluoride (100%) and 50 μM leupeptin (67.94%).



Similar content being viewed by others
Literature Cited
Blum H, Beier H, Gross H (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
Bouchara JP, Tronchin G, Larcher G, Cjabase D (1995) The search for virulence determinants in Aspergillus fumigatus. Trends Microbiol 3:327–330
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:680–685
Campos RA, Arruda W, Boldo JT, Da Silva MV, de Barros NM, de Azevedo JL, et al. (2005) Boophilus microplus infection by Beauveria amorpha and Beauveria bassiana: SEM analysis and regulation of subtilisin-like proteases and chitinases. Curr Microbiol 50:257–261
Chakrabarti SK, Matsumura N, Ranu RS (2000) Purification and characterization of an extracellular alkaline serine protease from Aspergillus terreus (IJIRA 6.2). Curr Microbiol 40:239–244
Escott GM, Hearn VM, Adams DJ (1998) Inducible chitinolytic system of Aspergillus fumigatus. Microbiology 144:1575–1581
Francheschini M, Guimarães AP, Camassola M, Frazz AP, Baratto C, Kogler V, et al. (2001) Biotecnologia aplicada ao controle biológico. Biotecnolog Ciênc Desenvolv 23:32–37
Franco OL, Rigden DJ, Melo FR, Bloch Jr C, Silva CP, Grossi de Sa MF (2000) Activity of wheat α-amylase inhibitors towards bruchid α-amylases and structural explanation of observed specificities. Eur J Biochem 267:2166–2173
Khan A, Willians K, Molloy MP, Nevalainen H (2003) Purification and characterization of a serine protease and chitinases from Paecilomyces lilacinus and detection of chitinase activity on 2D gels. Protein Expr Purif 32:210–220
Kogan TV, Jadoun J, Mittelman L, Hirschberg K, Osherov N (2004) Involvement of secreted Aspergillus fumigatus proteases in disruption of the actin fiber cytoskeleton and loss of focal adhesion sites in infected A549 lung pneumocytes. J Infect Dis 189:1965–1973
Kramer KJ, Muthukrishnan S (1997) Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem Mol Biol 11:887–900
Krikstaponis A, Lugauskas A, Trackzyk EK, Prazmo S, Dutkiewiez J (2001) Enzymatic activities of Aspergillus fumigatus strains isolated from the air at waste landfills. Ann Agric Environ Med 8:227–234
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of the bacteriophage T4. Nature (Lond) 227:680–685
Maurer KH (2004). Detergent proteases. Curr Opin Biotechnol 15:330–334
Noronha EF, Lima BD, Sá CM, Felix CR (2002) Heterologous production of Aspergillus fumigatus keratinase in Pichia pastoris. World J Microbiol Biotechnol 18:563–568
Palmieri G, Bianco C, Cennamo G, Giardina P, Marino G, Monti M, et al. (2001) Purification, characterization, and functional role of a novel extracellular protease from Pleurotus ostreatus. Appl Environ Microbiol 67:2754–2759
Petinate SDG, Branquinha MH, Coelho RRR, Vermelho AB, De Simone SG (1999) Purification and partial characterization of an extracellular serne-proteinase of Streptomyces cyaneus isolated from Brazilian cerrado soil. J Appl Microbiol 87:557–563
Punja ZK, Utkhede RS (2003) Using fungi and yeasts to manage vegetable crop diseases. Trends Biotechnol 21:400–407
Ra MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Molec Biol Rev 62:597–635
Santos RMDB, Firmino AAP, Sá CM, Félix CR (1996). Keratinolytic activity of Aspergillus fumigatus Fresenius. Curr Microbiol 33:364–370
Silva CHC (1997) Purificação e caracterização de uma β-xilanase de Aspergillus fumigatus Fresenius crescido em meio sólido. (1997). Master’s thesis. Universidade de Brasilia, Brasília DF, Brazil
Singh SR, Rachie KO (1985) Cowpea research, production and utilization. Chichester, Wiley
Tomschy A, Brugger R, Lehmann M, Syendsen A, Vogel K, Kostrewa D, et al. (2002) Engineering of phytase for improved activity at low pH. Appl Environ Microbiol 68:1907–1913
Ulhoa CJ, Peberdy JF (1992) Purification and some properties of the extracellular chitinase produced by Trichoderma harzianum. Enzyme Microb Technol 14:236–240
Viterbo A, Harel M, Chet I (2004). Isolation of two aspartyl proteases from Trichoderma asperellum expressed during colonization of cucumber roots. FEMS Microbiol Lett 238:151–154
Xia G, Jin C, Zhou J, Yang S, Zhang S, Jin C (2001). A novel chitinase having a unique mode of action from Aspergillus fumigatus YJ-407. Eur J Biochem 268:4079–4085
Ximenes EA (1996). Estudos da β-glucosidase de Aspergillus fumigatus Fresenius. (1996). Master’s thesis. UnB Brasília DF
Acknowledgments
This work was supported by a grant research from the Universidade Católica de Brasília. J. Pereira acknowledges C. Bloch Jr. for spectrometric analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, J.L., Franco, O.L. & Noronha, E.F. Production and Biochemical Characterization of Insecticidal Enzymes from Aspergillus fumigatus Toward Callosobruchus maculatus . Curr Microbiol 52, 430–434 (2006). https://doi.org/10.1007/s00284-005-0192-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-005-0192-x