Abstract
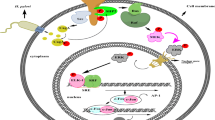
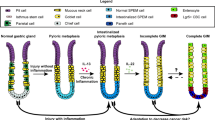
Accumulating evidence indicates that inflammation plays a critical role in cancer development. Cyclooxygenase-2 (COX-2) is a rate-limiting enzyme for prostanoid biosynthesis, including prostaglandin E2 (PGE2), and plays a key role in both inflammation and cancer. It has been demonstrated that inhibition of COX-2 and PGE2 receptor signaling results in the suppression of tumor development in a variety of animal models. However, the molecular mechanisms underlying COX-2/PGE2-associated inflammation in carcinogenesis have not yet been fully elucidated. In order to study the role of PGE2-associated inflammatory responses in tumorigenesis, it is important to use in vivo mouse models that recapitulate human cancer development from molecular mechanisms with construction of tumor microenvironment. We have developed a gastritis model (K19-C2mE mice) in which an inflammatory microenvironment is constructed in the stomach via induction of the COX-2/PGE2 pathway. We also developed a gastric cancer mouse model (Gan mice) in which the mice develop inflammation-associated gastric tumors via activation of both the COX-2/PGE2 pathway and Wnt signaling. Expression analyses using these in vivo models have revealed novel mechanisms of the inflammatory responses underlying gastric cancer development. PGE2-associated inflammatory responses activate epidermal growth factor receptor (EGFR) signaling through the induction of EGFR ligands and ADAMs that release EGFR ligands from the cell membrane. In Gan mice, a combination treatment with EGFR and COX-2 inhibitors significantly suppresses gastric tumorigenesis. Moreover, PGE2-associated inflammation downregulates tumor suppressor microRNA, miR-7, in gastric cancer cells, which suppresses epithelial differentiation. These results indicate that PGE2-associated inflammatory responses promote in vivo gastric tumorigenesis via several different molecular mechanisms.





Similar content being viewed by others
References
Kuper H, Adami HO, Trichopoulos D (2000) Infections as a major preventable cause of human cancer. J Intern Med 248:171–183
Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118:3030–3044
Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M (2010) Tobacco smoke promotes lung tumorigenesis by triggering IKKβ- and JNK1-dependent inflammation. Cancer Cell 17:89–97
Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M (2010) Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140:197–208
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899
Wang D, DuBois RN (2010) Eicosanoids and cancer. Nat Rev Cancer 10:181–193
Wang D, DuBois RN (2010) The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 29:781–788
Li HJ, Reinhardt F, Herschman HR, Weinberg RA (2012) Cancer-stimulated mesenchymal stem cells create a carcinoma stem-cell niche via prostaglandin E2 signaling. Cancer Discov 2:840–855
Oshima H, Oguma K, Du YC, Oshima M (2009) Prostaglandin E2, Wnt, and BMP in gastric tumor mouse models. Cancer Sci 100:1779–1785
Oshima H, Oshima M (2010) Mouse models of gastric tumors: Wnt activation and PGE2 induction. Pathol Int 60:599–607
Thun MJ, Namboodiri MM, Heath CW Jr (1991) Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 325:1593–1596
Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE (1995) Aspirin and the risk of colorectal cancer in women. N Engl J Med 333:609–614
Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ (1993) Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 328:1313–1316
Oshima M, Taketo MM (2002) COX selectivity and animal models for colon cancer. Curr Pharm Des 8:1021–1034
Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM (1996) Suppression of intestinal polyposis in Apc∆716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87:803–809
Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, Tiano HF, Morham SG, Smithies O, Langenbach R (2000) Genetic disruption of Ptgs-1, as well as of Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res 60:4705–4708
Yoshimatsu K, Altorki NK, Golijanin D, Zhang F, Jakobsson PJ, Dannenberg AJ, Subbaramaiah K (2001) Inducible prostaglandin E synthase is overexpressed in non-small cell lung cancer. Clin Cancer Res 7:2669–2674
van Rees BP, Sivula A, Thoren S, Yokozaki H, Jalobsson PJ, Offerhaus GJ, Ristimaki A (2003) Expression of microsomal prostaglandin E synthase-1 in intestinal gastric adenocarcinoma and in gastric cancer cell lines. Int J Cancer 107:551–556
Nakanishi M, Montrose DC, Clark P, Nambiar PR, Belinsky GS, Claffey KP, Xu D, Rosenberg DW (2008) Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res 68:3251–3259
Nakanishi M, Menoret A, Tanaka T, Miyamoto S, Montrose DC, Vella AT, Rosenberg DW (2011) Selective PGE2 suppression inhibits colon carcinogenesis and modifies local mucosal immunity. Cancer Prev Res 4:1198–1208
Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Natumiya S, Oshima M, Taketo MM (2001) Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc∆716 knockout mice. Nat Med 7:1048–1051
Seno H, Oshima M, Ishikawa TO, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM (2002) Cyclooxygenase 2- and prostaglandin E2 receptor EP2-dependent angiogenesis in Apc∆716 mouse intestinal polyps. Cancer Res 62:506–511
Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS (2005) Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin–β-catenin signaling axis. Science 310:1504–1510
Balkwill F (2009) Tumor necrosis factor and cancer. Nat Rev Cancer 9:361–371
Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, Kollias G, Balkwill F (1999) Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nat Med 5:828–831
Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR (2004) Expression of both TNF-α receptor subtypes is essential for optimal skin tumor development. Oncogene 23:1902–1910
Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N (2008) Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118:560–570
Oshima H, Oshima M (2012) The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models. J Gastroenterol 47:97–106
Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M (2004) IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118:285–296
Heikkila K, Ebrahim S, Lawlor DA (2008) Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer 44:937–945
Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitala S, Matthews V, Schmid RM, Kirchner T, Arkan MC, Ernst M, Greten FR (2009) gp130-mediated STAT3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15:91–102
Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M (2009) IL-6 and STAT3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15:103–113
Gregorieff A, Clevers H (2005) Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 19:877–890
Taketo MM (2006) Wnt signaling and gastrointestinal tumorigenesis in mouse models. Oncogene 25:7522–7530
Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M (1995) Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci USA 92:4482–4486
Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM (1999) Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J 18:5931–5942
Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, Groden J, Lowy AM (2002) β-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res 62:3503–3506
Woo DK, Kim HS, Lee HS, Kang YH, Yang HK, Kim WH (2001) Altered expression and mutation of β-catenin gene in gastric carcinomas and cell lines. Int J Cancer 95:108–113
Cheng XX, Wang ZC, Chen XY, Sun Y, Kong QY, Liu J, Li H (2005) Correlation of Wnt-2 expression and β-catenin intracellular accumulation in Chinese gastric cancers: relevance with tumour dissemination. Cancer Lett 223:339–347
Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M (2006) Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology 131:1086–1095
Park WS, Oh RR, Park JY, Lee SH, Shin MS, Kim YS, Kim SY, Lee HK, Kim PJ, Oh ST, Yoo NJ, Lee JY (1999) Frequent somatic mutations of the β-catenin gene in intestinal-type gastric cancer. Cancer Res 59:4257–4260
Ebert MP, Fei G, Kahmann S, Muller O, Yu J, Sung JJ, Malfertheiner P (2002) Increased β-catenin mRNA levels and mutational alterations of the APC and β-catenin gene are present in intestinal-type gastric cancer. Carcinogenesis 23:87–91
Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M (2008) Activated macrophages promote Wnt signalling through tumour necrosis factor-α in gastric tumour cells. EMBO J 27:1671–1681
Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M (1997) Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res 57:1276–1280
Al-Marhoon MS, Nunn S, Soames RW (2004) CagA + Helicobacter pylori induces greater levels of prostaglandin E2 than cagA − strains. Prostaglandins Other Lipid Mediat 73:181–189
Sun WH, Yu Q, Shen H, Qu XL, Cao DZ, Yu T, Qian C, Zhu F, Sun YL, Fu XL, Su H (2004) Roles of Helicobacter pylori infection and cyclooxygenase-2 expression in gastric carcinogenesis. World J Gastroenterol 10:2809–2813
Oshima H, Oshima M, Inaba K, Taketo MM (2004) Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J 23:1669–1678
Oshima M, Ohima H, Matsunaga A, Taketo MM (2005) Hyperplastic gastric tumors with spasmolytic polypeptide-expressing metaplasia caused by tumor necrosis factor-α-dependent inflammation in cyclooxygenase-2/microsomal prostaglandin E synthase-1 transgenic mice. Cancer Res 65:9147–9151
Yamaguchi H, Goldenring JR, Kaminishi M, Lee JR (2002) Identification of spasmolytic polypeptide-expressing metaplasia (SPEM) in remnant gastric cancer and surveillance post-gastrectomy biopsies. Dig Dis Sci 47:573–578
Halldorsdottir AM, Sigurdardottrir M, Jonasson JG, Oddsdottir M, Magnusson J, Lee JR, Goldenring JR (2003) Spasmolytic polypeptide-expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci 48:431–441
Goldenring JR, Nomura S (2009) Insight into the development of preneoplastic metaplasia: spasmolytic polypeptide-expressing metaplasia and oxyntic atrophy. In: Wang TC, Fox JG, Giraud AS (eds) The biology of gastric cancer. Springer, New York, pp 361–375
Nomura S, Baxter T, Yamaguchi H, Leys C, Vartapetian AB, Fox JG, Lee JR, Wang TC, Goldenring JR (2004) Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology 127:582–594
Kang W, Rathinavelu S, Samuelson LC, Merchang JL (2005) Interferon γ induction of gastric mucous neck cell hypertrophy. Lab Invest 85:702–715
Judd LM, Alderman BM, Howlett M, Shulkes A, Dow C, Moverley J, Grail D, Jenkins BJ, Ernst M, Giraud AS (2004) Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology 126:196–207
Guo X, Oshima H, Kitamura T, Taketo MM, Oshima M (2008) Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem 283:19864–19871
Itadani H, Oshima H, Oshima M, Kotani H (2009) Mouse gastric tumor models with prostaglandin E2 pathway activation show similar gene expression profiles to intestinal-type human gastric cancer. BMC Genomics 10:615
Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba E, Saya H (2011) CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of xc− and thereby promotes tumor growth. Cancer Cell 19:387–400
Ishimoto T, Oshima H, Oshima M, Kai K, Torii R, Masuko T, Baba H, Saya H, Nagano O (2010) CD44(+) slow-cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci 101:673–678
Oshima H, Hioki K, Popivanova BK, Oguma K, van Rooijen N, Ishikawa T, Oshima M (2011) Prostaglandin E2 signaling and bacterial infection recruit tumor-promoting macrophages to mouse gastric tumors. Gastroenterology 140:596–607
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R (2004) Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118:229–241
Rakoff-Nahoum S, Medzhitov R (2007) Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 317:124–127
Chinen T, Komai K, Muto G, Morita R, Inoue N, Yoshida H, Sekiya T, Yoshida R, Nakamura K, Takayanagi R, Yoshimura A (2010) Prostaglandin E2 and SOCS1 have a role in intestinal immune tolerance. Nature Commun 2:190
Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN (2005) Cyclooxigenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol 2:254–266
Roberts RB, Min L, Washington MK, Olsen SJ, Settle SH, Coffey RJ, Threadgill DW (2002) Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci USA 99:1521–1526
Torrance CJ, Jackson PE, Montgomery E, Kinzler KW, Vogelstein B, Wissner A, Nunes M, Frost P, Discafani CM (2006) Combinatorial chemoprevention of intestinal neoplasia. Nat Med 6:1024–1028
Buchanan FG, Holla V, Katkuri S, Matta P, DuBois RN (2008) Targeting cyclooxygenase-2 and the epidermal growth factor receptor for the prevention and treatment of intestinal cancer. Cancer Res 67:9380–9388
Buchanan FG, Wang D, Bargiacchi F, DuBois RN (2003) Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem 278:35451–35457
Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN (2006) Role of β-arrestin1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci USA 103:1492–1497
Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS (2002) Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med 8:289–293
Shao J, Lee SB, Guo H, Evers BM, Sheng H (2003) Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Res 63:5218–5223
Subbaramaiah K, Benezra R, Hudis C, Dannenberg AJ (2008) Cyclooxygenase-2-derived prostaglandin E2 stimulates Id-1 transcription. J Biol Chem 283:33955–33968
Al-Salihi MA, Ulmer SC, Doan T, Nelson CD, Crotty T, Prescott SM, Stafforini DM, Topham MK (2007) Cyclooxygenase-2 transactivates the epidermal growth factor receptor through specific E-prostanoid receptors and tumor necrosis factor-α converting enzyme. Cell Signal 19:1956–1963
Oshima H, Popivanova BK, Oguma K, Dong D, Ishikawa T, Oshima M (2011) Activation of epidermal growth factor receptor signaling by the prostaglandin E2 receptor EP4 pathway during gastric tumorigenesis. Cancer Sci 102:713–719
Mochizuki S, Okada Y (2007) ADAMs in cancer cell proliferation and progression. Cancer Sci 98:621–628
Oshima H, Itadani H, Kotani H, Taketo MM, Oshima M (2009) Induction of prostaglandin E2 pathway promotes gastric hamartoma development with suppression of bone morphogenetic protein signaling. Cancer Res 69:2729–2733
Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signaling network. Nat Rev Mol Cell Biol 2:127–137
Mann JR, Backlund MG, Buchanan FG, Daikoku T, Holla VR, Rosenberg DW, Dey SK, DuBois RN (2006) Repression of prostaglandin dehydrogenase by epidermal growth factor and snail increases prostaglandin E2 and promotes cancer progression. Cancer Res 66:6649–6656
Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, Lawrence E, Dannenberg AJ, Lovgren AK, Luo G, Pretlow TP, Newman RA, Willis J, Dawson D, Markowitz SD (2006) 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci USA 103:12098–12102
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Esquela-Kerscher A, Slack FJ (2006) Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6:259–269
Ventura A, Jacks T (2009) MicroRNAs and cancer: short RNAs go a long way. Cell 136:586–591
Di Leva G, Croce CM (2010) Roles of small RNAs in tumor formation. Trends Mol Med 16:257–267
Sonkoly E, Pivarcsi A (2011) MicroRNAs in inflammation and response to injuries induced by environmental pollution. Mutat Res 717:46–53
O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D (2007) MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA 104:1604–1609
Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM (2007) Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 179:5082–5089
Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K (2010) STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell 39:493–506
Kong D, Piao YS, Yamashita S, Oshima H, Oguma K, Fushida S, Fujimura T, Minamoto T, Seno H, Yamada Y, Satou K, Ushijima T, Ishikawa T, Oshima M (2012) Inflammation-induced repression of tumor suppressor miR-7 in gastric tumor cells. Oncogene 31:3949–3960
Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103:2257–2261
Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo YY (2009) p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci USA 106:3207–3212
Kefas B, Godlewski J, Comeau L, Li Y, Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S, Purow B (2008) MicroRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res 68:3566–3572
Reddy SD, Ohshiro K, Rayala SK, Kumar R (2008) MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase 1 and regulates its functions. Cancer Res 68:8195–8200
Webster RJ, Giles KM, Price KJ, Zhang PM, Mattick JS, Leedman PJ (2009) Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J Biol Chem 284:5731–5741
Jiang L, Liu X, Chen Z, Jin Y, Heidbreder CE, Kolokythas A, Wang A, Dai Y, Zhou X (2010) MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells. Biochem J 432:199–205
Saydam O, Senol O, Wurdinger T, Mizrak A, Ozdener GB, Stemmer-Rachamimov AO, Yi M, Stephens RM, Krichevsky AM, Saydam N, Brenner GJ, Breakefield XO (2011) miRNA-7 attenuation in schwannoma tumors stimulates growth by upregulating three oncogenic signaling pathways. Cancer Res 71:852–861
Acknowledgments
We thank Manami Watanabe for her excellent work in the series of Gan mouse studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on Inflammation and Cancer - Guest Editor: Takuji Tanaka
Rights and permissions
About this article
Cite this article
Oshima, H., Oshima, M. The role of PGE2-associated inflammatory responses in gastric cancer development. Semin Immunopathol 35, 139–150 (2013). https://doi.org/10.1007/s00281-012-0353-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-012-0353-5