Abstract
Purpose
This Phase I study evaluated the safety, tolerability, food effects, pharmacodynamics, and pharmacokinetics of donafenib in patients with advanced solid tumours.
Methods
Eligible patients received a single dose of donafenib (50 mg, 100 mg, 200 mg, 300 mg, or 400 mg) and were then observed over a 7-day period; thereafter, each patient received the corresponding dose of donafenib twice daily for at least 4 weeks. Safety assessment and pharmacokinetic sampling were performed for all patients at the given time points; preliminary tumour response was also assessed.
Results
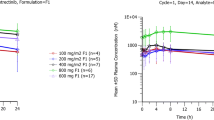
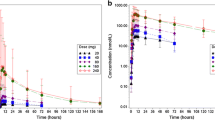
Twenty-five patients were enrolled in this study. Gastrointestinal reactions were the most common treatment-related adverse event, followed by skin toxicity. The maximum tolerated dose (MTD) was 300 mg bid. The dose-limiting toxicities (DLTs) were grade 3 diarrhoea and fatigue at 300 mg bid and grade 3 skin toxicity at 400 mg bid. In the dose range of 100 ~ 400 mg, T1/2 and AUC0–t after multiple doses were 26.9 ~ 30.2 h and 189 ~ 356 h*μg/mL, respectively. Food did not have a significant effect on the pharmacokinetics of donafenib. Twenty-one patients were assessed for efficacy, and two patients achieved a partial response according to Response Evaluation Criteria in Solid Tumors (RECIST), with a disease control rate of 57.1%.
Conclusion
Oral donafenib was generally well tolerated and appeared to provide some clinical benefits; adverse events were manageable. Based on the results of this study, oral donafenib at 200 mg ~ 300 mg twice daily is recommended for further studies.



Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the concentration–time curve
- AUC0–12 :
-
Area under the concentration–time curve for 0–12 h
- AUC0–24 h :
-
Area under the plasma concentration–time cuvre from 0 to 24 h
- AUC0–t :
-
Area under the concentration–time curve from time zero to 120 h
- AUC 0–∞ :
-
Area under the plasma concentration–time curve from time zero to infinity
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- CR:
-
Complete response
- CTCAE:
-
The common terminology criteria for adverse events
- C max :
-
Peak concentration in plasma
- C avg :
-
Average plasma drug concentration
- C min :
-
Minimum plasma drug concentration
- Cl/F:
-
Apparent total clearance of the drug from plasma after oral administration
- CV:
-
Coefficient of variation
- DF:
-
Degree of fluctuation
- R :
-
Ratio of accumulation factor
- DLT:
-
Dose-limited toxicity
- ECOG:
-
Eastern Cooperative Oncology Group
- FDA:
-
Food and Drug Administration
- GCP:
-
Good clinical practice
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- ICH:
-
The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use
- MRT0–∞ :
-
Mean residence time from time zero to infinity
- MTD:
-
Maximum tolerated dose
- NCI CTC:
-
National Cancer Institute common terminology criteria
- NCA:
-
Non-compartment analysis
- PDGFR:
-
Platelet-derived growth factor receptor
- PR:
-
Partial response
- PD:
-
Progressive disease
- RE:
-
Relative error
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- RSD:
-
Relative standard deviation
- SD:
-
Stable disease
- Tmax:
-
Time to Cmax
- t 1/2 :
-
Half-life
- TBIL:
-
Total bilirubin
- ULN:
-
Upper limit of normal
- VEGFR:
-
Vascular endothelial growth factor receptor
- Vz/F :
-
Apparent volume of distribution during terminal phase after non-intravenous administration
References
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, Group SIS (2008) Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine 359(4):378–390. https://doi.org/10.1056/NEJMoa0708857
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, Group TS (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. The New England journal of medicine 356(2):125–134. https://doi.org/10.1056/NEJMoa060655
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30. https://doi.org/10.3322/caac.21332
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2):115–132. https://doi.org/10.3322/caac.21338
Kirk GD, Lesi OA, Mendy M, Akano AO, Sam O, Goedert JJ, Hainaut P, Hall AJ, Whittle H, Montesano R (2004) The Gambia Liver Cancer Study: infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology 39(1):211–219. https://doi.org/10.1002/hep.20027
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34. https://doi.org/10.1016/S1470-2045(08)70285-7
Parikh ND, Marshall VD, Singal AG, Nathan H, Lok AS, Balkrishnan R, Shahinian V (2017) Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: an analysis of the SEER-Medicare database. Hepatology 65(1):122–133. https://doi.org/10.1002/hep.28881
Leung HW, Liu CF, Chan AL (2016) Cost-effectiveness of sorafenib versus SBRT for unresectable advanced hepatocellular carcinoma. Radiat Oncol 11:69. https://doi.org/10.1186/s13014-016-0644-4
Parli CJ, McMahon RE (1973) The mechanism of microsomal deamination: heavy isotope studies. Drug Metab Dispos 1(1):337–341
Mutlib AE, Gerson RJ, Meunier PC, Haley PJ, Chen H, Gan LS, Davies MH, Gemzik B, Christ DD, Krahn DF, Markwalder JA, Seitz SP, Robertson RT, Miwa GT (2000) The species-dependent metabolism of efavirenz produces a nephrotoxic glutathione conjugate in rats. Toxicol Appl Pharmacol 169(1):102–113. https://doi.org/10.1006/taap.2000.9055
Mutlib AE (2008) Application of stable isotope-labeled compounds in metabolism and in metabolism-mediated toxicity studies. Chem Res Toxicol 21(9):1672–1689. https://doi.org/10.1021/tx800139z
Katsnelson A (2013) Heavy drugs draw heavy interest from pharma backers. Nat Med 19(6):656. https://doi.org/10.1038/nm0613-656
Pang X, Peng L, Chen Y (2017) Effect of N-methyl deuteration on pharmacokinetics and pharmacodynamics of enzalutamide. J Labelled Comp Radiopharm 60(9):401–409. https://doi.org/10.1002/jlcr.3516
Jiang J, Pang X, Li L, Dai X, Diao X, Chen X, Zhong D, Wang Y, Chen Y (2016) Effect of N-methyl deuteration on metabolism and pharmacokinetics of enzalutamide. Drug Des Dev Ther 10:2181–2191. https://doi.org/10.2147/DDDT.S111352
Nelson SD, Trager WF (2003) The use of deuterium isotope effects to probe the active site properties, mechanism of cytochrome P450-catalyzed reactions, and mechanisms of metabolically dependent toxicity. Drug Metab Dispos 31(12):1481–1498. https://doi.org/10.1124/dmd.31.12.1481
Sharma R, Strelevitz TJ, Gao H, Clark AJ, Schildknegt K, Obach RS, Ripp SL, Spracklin DK, Tremaine LM, Vaz AD (2012) Deuterium isotope effects on drug pharmacokinetics. I. System-dependent effects of specific deuteration with aldehyde oxidase cleared drugs. Drug Metab Dispos 40(3):625–634. https://doi.org/10.1124/dmd.111.042770
Zhong L, Hou C, Zhang L, Zhao J, Li F, Li W (2019) Synthesis of deuterium-enriched sorafenib derivatives and evaluation of their biological activities. Mol Divers 23(2):341–350. https://doi.org/10.1007/s11030-018-9875-7
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64(19):7099–7109. https://doi.org/10.1158/0008-5472.CAN-04-1443
Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, Bortolon E, Ichetovkin M, Chen C, McNabola A, Wilkie D, Carter CA, Taylor IC, Lynch M, Wilhelm S (2007) Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol 59(5):561–574. https://doi.org/10.1007/s00280-006-0393-4
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S (2006) Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 5(10):835–844. https://doi.org/10.1038/nrd2130
Huynh H, Nguyen TT, Chow KH, Tan PH, Soo KC, Tran E (2003) Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol 3:19. https://doi.org/10.1186/1471-230X-3-19
Yoshida T, Hisamoto T, Akiba J, Koga H, Nakamura K, Tokunaga Y, Hanada S, Kumemura H, Maeyama M, Harada M, Ogata H, Yano H, Kojiro M, Ueno T, Yoshimura A, Sata M (2006) Spreds, inhibitors of the Ras/ERK signal transduction, are dysregulated in human hepatocellular carcinoma and linked to the malignant phenotype of tumors. Oncogene 25(45):6056–6066. https://doi.org/10.1038/sj.onc.1209635
Poon RT, Lau C, Pang R, Ng KK, Yuen J, Fan ST (2007) High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: importance of tumor biomarker in ablative therapies. Ann Surg Oncol 14(6):1835–1845. https://doi.org/10.1245/s10434-007-9366-z
Wada H, Nagano H, Yamamoto H, Yang Y, Kondo M, Ota H, Nakamura M, Yoshioka S, Kato H, Damdinsuren B, Tang D, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Nakamori S, Sakon M, Dono K, Wakasa K, Monden M (2006) Expression pattern of angiogenic factors and prognosis after hepatic resection in hepatocellular carcinoma: importance of angiopoietin-2 and hypoxia-induced factor-1 alpha. Liver Int 26(4):414–423. https://doi.org/10.1111/j.1478-3231.2006.01243.x
Stock P, Monga D, Tan X, Micsenyi A, Loizos N, Monga SP (2007) Platelet-derived growth factor receptor-alpha: a novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther 6(7):1932–1941. https://doi.org/10.1158/1535-7163.MCT-06-0720
Wang J, Lu BH, Dai XJ, Zhang YF, Chen XY, Zhong DF (2017) Simultaneous determination of donafenib and its N-oxide metabolite in human plasma by liquid chromatography-tandem mass spectrometry. Yao Xue Xue Bao 52(3):443–448
Furuse J, Ishii H, Nakachi K, Suzuki E, Shimizu S, Nakajima K (2008) Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci 99(1):159–165. https://doi.org/10.1111/j.1349-7006.2007.00648.x
Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ (2005) Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43–9006, in patients with advanced, refractory solid tumors. Clin Cancer Res 11(15):5472–5480. https://doi.org/10.1158/1078-0432.CCR-04-2658
Strumberg D, AA, Piccart P, et al (2003) Final report of the multiple single agent phase I clinical trials of the novel Raf kinase inhibitor BAY 43-9006 in patients with refractory solid tumors [Abstract]. Proc Am Soc Clin Oncol 22 (203)
Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, Taylor I, Moscovici M, Saltz LB (2006) Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24(26):4293–4300. https://doi.org/10.1200/JCO.2005.01.3441
Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG, Schwartz B, Awada A, Voigtmann R, Scheulen ME, Seeber S (2005) Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol 23(5):965–972. https://doi.org/10.1200/JCO.2005.06.124
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D, Group CS (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381(9863):303–312. https://doi.org/10.1016/S0140-6736(12)61900-X
Funding
The work was supported by Zelgen Biopharmaceuticals and grants from National Science and Technology Specific Projects of China (No. 2017ZX09304023), the Major Specific Project of Sichuan province (No. 20ZDYF3127), and the National Science and Technology Specific Projects of China (No. 2018ZX09201018-020).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Qiu, M., Wang, S. et al. A Phase I dose-escalation, pharmacokinetics and food-effect study of oral donafenib in patients with advanced solid tumours. Cancer Chemother Pharmacol 85, 593–604 (2020). https://doi.org/10.1007/s00280-020-04031-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04031-1