Abstract
Objective
This study aimed to reveal the efficacy and safety profiles of 4-weekly cabazitaxel in patients with castration-resistant prostate cancer (CRPC).
Methods
The study included 62 Japanese patients who were treated for CRPC with ≥ 2 courses of cabazitaxel between 2014 and 2017. The oncological outcomes and adverse events were compared between 16 (25.8%) and 46 (74.2%) men who were treated with standard 3-weekly and alternative 4-weekly regimens, respectively.
Results
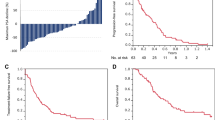
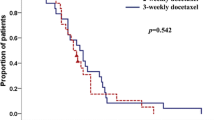
The prostate-specific antigen (PSA) response was comparable between the 3-weekly and 4-weekly regimens (median [interquartile range]: − 9.9% [− 64.5 to 13.0%] and − 30.7% [− 52.8 to 10.9%], P = 0.89), respectively. For patients on the 4-weekly regimen, the risks of progression (hazard ratio [HR], 95% confidence interval [CI] 1.27, 0.71–2.43, P = 0.44), treatment failure (HR, 95% CI 0.84, 0.48–1.55, P = 0.57) and any-cause mortality (HR, 95% CI 1.09, 0.58–2.17, P = 0.79) were comparable to those for patients on the 3-weekly regimen. The incidences of severe adverse events were also similar between the 3-weekly and 4-weekly regimens.
Conclusions
3-weekly and 4-weekly regimens of cabazitaxel showed similar efficacy and safety profiles in a real-world clinical setting. These data suggest that a 4-weekly regimen may be acceptable for selected patients.

Similar content being viewed by others
References
Shiota M, Yokomizo A, Eto M (2016) Taxane chemotherapy for Hormone-Naïve prostate cancer with its expanding role as breakthrough strategy. Front Oncol 5:304
Shiota M, Eto M (2016) Current status of primary pharmacotherapy and future perspectives toward upfront therapy for metastatic hormone-sensitive prostate cancer. Int J Urol 23:360–369
Komura K, Sweeney CJ, Inamoto T, Ibuki N, Azuma H, Kantoff PW (2018) Current treatment strategies for advanced prostate cancer. Int J Urol 25:220–231
Fujimoto N (2016) Novel agents for castration-resistant prostate cancer: early experience and beyond. Int J Urol 23:114–121
de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO, Investigators TROPIC (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376:1147–1154
Bahl A, Oudard S, Tombal B, Ozgüroglu M, Hansen S, Kocak I, Gravis G, Devin J, Shen L, de Bono JS, Sartor AO, Investigators TROPIC (2013) Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann Oncol 24:2402–2408
Oudard S, Fizazi K, Sengeløv L, Daugaard G, Saad F, Hansen S, Hjälm-Eriksson M, Jassem J, Thiery-Vuillemin A, Caffo O, Castellano D, Mainwaring PN, Bernard J, Shen L, Chadjaa M, Sartor O (2017) Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J Clin Oncol 35:3189–3197
Eisenberger M, Hardy-Bessard AC, Kim CS, Géczi L, Ford D, Mourey L, Carles J, Parente P, Font A, Kacso G, Chadjaa M, Zhang W, Bernard J, de Bono J (2017) Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol 35:3198–3206
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M, Prostate Cancer Clinical Trials Working Group (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26:1148–1159
Climent MÁ, Pérez-Valderrama B, Mellado B, Fernández Parra EM, Fernández Calvo O, Ochoa de Olza M, Muinelo Romay L, Anido U, Domenech M, Hernando Polo S, Arranz Arija JÁ, Caballero C, Juan Fita MJ, Castellano D (2017) Weekly cabazitaxel plus prednisone is effective and less toxic for ‘unfit’ metastatic castration-resistant prostate cancer: phase II Spanish Oncology Genitourinary Group (SOGUG) trial. Eur J Cancer 87:30–37
Yachnin J, Gilje B, Thon K, Johansson H, Brandberg Y, Panaretakis T, Ullén A (2018) Weekly versus 3-weekly cabazitaxel for the treatment of castration-resistant prostate cancer: a randomised phase II trial (ConCab). Eur J Cancer 97:33–40
Clément-Zhao A, Auvray M, Aboudagga H, Blanc-Durand F, Angelergues A, Vano YA, Mercier F, El Awadly N, Verret B, Thibault C, Oudard S (2018) Safety and efficacy of 2-weekly cabazitaxel in metastatic castration-resistant prostate cancer. BJU Int 121:203–208
Acknowledgements
We would like to acknowledge the contributions of the following collaborators: Akito Yamaguchi (Harasanshin Hospital, Fukuoka), Takashi Dejima (Kyushu Central Hospital, Fukuoka), Satoshi Otsubo (Kitakyushu Municipal Medical Center, Kitakyushu), Akio Tsutsui (JCHO Kyushu Hospital, Kitakyushu), and Yoshifumi Hori (Miyazaki Prefectural Miyazaki Hospital, Miyazaki). We thank Anne M. O’Rourke, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This work was supported by JSPS KAKENHI Grant (17K11145).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
IRB approval from Kyushu University Hospital, National Hospital Organization Kyushu Cancer Center, Harasanshin Hospital, Oita Prefectural Hospital, National Hospital Organization Kyushu Medical Center, Kyushu Central Hospital, Kitakyushu Municipal Medical Center, Japanese Red Cross Fukuoka Hospital, JCHO Kyushu Hospital, and Miyazaki Prefectural Miyazaki Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shiota, M., Nakamura, M., Yokomizo, A. et al. Efficacy and safety of 4-weekly cabazitaxel for castration-resistant prostate cancer: a multi-institutional study. Cancer Chemother Pharmacol 84, 561–566 (2019). https://doi.org/10.1007/s00280-019-03874-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03874-7