Abstract
Purpose
The phase III MARIANNE study investigated single-agent trastuzumab emtansine (T-DM1) and combination T-DM1 plus pertuzumab as the first-line treatment for human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC). Pharmacokinetic properties of T-DM1 and pertuzumab in these patients and the potential for drug–drug interactions (DDIs) were assessed.
Methods
Pharmacokinetic samples of T-DM1-related analytes (T-DM1 conjugate, total trastuzumab, DM1) and pertuzumab were analyzed. Observed pharmacokinetic data were summarized for all analytes. Historical population pharmacokinetic models for T-DM1 conjugate and pertuzumab in HER2-positive MBC were used to derive empirical Bayes estimates of pharmacokinetic parameters.
Results
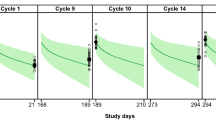
In MARIANNE (N = 375), mean ± standard deviation population pharmacokinetic model-predicted Cycle 1 Cmax for T-DM1 conjugate was 74.4 ± 10.1 µg/mL, Cycle 1 Ctrough was 1.34 ± 0.802 µg/mL, and area under the concentration–time curve from time zero to infinity after first dose (AUCinf) was 338 ± 69.5 µg*day/mL. These values were similar to other T-DM1 studies. Pharmacokinetics of T-DM1 conjugate and other analytes (total trastuzumab, DM1) were similar with or without pertuzumab. In the pertuzumab plus T-DM1 arm, mean model-predicted Cycle 1 pertuzumab Cmax, Ctrough, and AUCinf were 276 ± 50.0 µg/mL, 64.8 ± 17.9 μg/mL, and 4470 ± 1360 µg*day/mL, respectively. These values were similar to other pertuzumab studies.
Conclusions
Based on the population pharmacokinetic analysis of T-DM1 conjugate and pertuzumab, pharmacokinetics are similar across different lines of treatment and stages of disease including previously untreated MBC patients, and no DDIs were identified for combined use of T-DM1 and pertuzumab.




Similar content being viewed by others
References
Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX (2011) Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 128(2):347–356. https://doi.org/10.1007/s10549-010-1090-x
Lewis Phillips GD, Li G, Dugger DL et al (2008) Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 68(22):9280–9290. https://doi.org/10.1158/0008-5472.CAN-08-1776
Barok M, Tanner M, Koninki K, Isola J (2011) Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett 306(2):171–179. https://doi.org/10.1016/j.canlet.2011.03.002
Krop IE, Kim SB, González-Martín A et al (2014) Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 15(7):689–699. https://doi.org/10.1016/S1470-2045(14)70178-0
Verma S, Miles D, Gianni L et al (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367(19):1783–1791. https://doi.org/10.1056/NEJMoa1209124
Genentech, Inc., South San Francisco, CA (2016) KADCYLA® (ado-trastuzumab emtansine) for injection [package insert]
Baselga J, Swain SM (2009) Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 9:463–475
Scheuer W, Friess T, Burtscher H et al (2009) Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 69:9330–9336
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 4.2018. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed Feb 14 2019
Perez EA, Barrios C, Eiermann W et al (2017) Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the Phase III MARIANNE study. J Clin Oncol 35(2):141–148
Montemurro F, Ellis P, Anton A et al (2019) Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: primary results from the KAMILLA study cohort 1. Eur J Cancer 109:92–102
Bonotto M, Gerratana L, Iacono D, Minisini AM, Rihawi K, Fasola G, Puglisi F (2015) Treatment of metastatic breast cancer in a real-world scenario: is progression-free survival with first line predictive of benefit from second and later lines? Oncologist 20(7):719–724
Bakker JL, Wever K, van Waesberghe JH, Beeker A, Meijers-Heijboer H, Konings IR, Verheul HM (2015) What is the benefit of treatment with multiple lines of chemotherapy for patients with metastatic breast cancer? A retrospective cohort study. Cancer Epidemiol 39(6):848–853
Garg A, Quartino A, Li J et al (2014) Population pharmacokinetic and covariate analysis of pertuzumab, a HER2-targeted monoclonal antibody, and evaluation of a fixed, non-weight-based dose in patients with a variety of solid tumors. Cancer Chemother Pharmacol 74(4):819–829. https://doi.org/10.1007/s00280-014-2560-3
Girish S, Gupta M, Wang B et al (2012) Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol 69(5):1229–1240. https://doi.org/10.1007/s00280-011-1817-3
Lu D, Girish S, Gao Y et al (2014) Population pharmacokinetics of trastuzumab emtansine (T-DM1), a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer: clinical implications of the effect of covariates. Cancer Chemother Pharmacol 74(2):399–410. https://doi.org/10.1007/s00280-014-2500-2
Kim JH, Min SJ, Jang HJ, Cho JW, Kim SH, Kim HS (2015) Comparison of RECIST 1.0 and RECIST 1.1 in patients with metastatic cancer: a pooled analysis. J Cancer 6(4):387–393
Brendel K, Comets E, Laffont C, Mentré F (2010) Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn 37(1):49–65
Swain SM, Kim S-B, Cortés J et al (2013) Overall survival benefit with pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer in CLEOPATRA, a randomised Phase 3 study. Lancet Oncol 14(6):461–471. https://doi.org/10.1016/S1470-2045(13)70130-X
Gianni L, Pienkowski T, Im YH et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13(1):25–32. https://doi.org/10.1016/S1470-2045(11)70336-9
Chen S-C, Quartino A, Polhamus D et al (2017) Population pharmacokinetics and exposure-response of trastuzumab emtansine in advanced breast cancer previously treated with ≥ 2 HER2-targeted regimens. Br J Clin Pharmacol 83(12):2767–2777
Lu D, Burris HA 3rd, Wang B et al (2012) Drug interaction potential of trastuzumab emtansine (T-DM1) combined with pertuzumab in patients with HER2-positive metastatic breast cancer. Curr Drug Metab 13(7):911–922
Ryman JT, Meibohm B (2017) Pharmacokinetics of monoclonal antibodies. CPT Pharmacomet Syst Pharmacol 6(9):576–588. https://doi.org/10.1002/psp4.12224
Mould DR (2015) The pharmacokinetics of biologics: a primer. Dig Dis 33(Suppl 1):61–69
Lu D, Sahasranaman S, Zhang Y, Girish S (2013) Strategies to address drug interaction potential for antibody-drug conjugates in clinical development. Bioanalysis 5(9):1115–1130
Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M (2009) Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 69(24):9330–9336
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX (2004) Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5(4):317–328
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82(1):17–20
Acknowledgements
The authors acknowledge the investigators, patients, and their families who participated in these clinical trials. The authors are grateful for the assistance of Jia Kang, PhD, from Metrum Research Group, for her assistance in reviewing the data presented in this manuscript.
Funding
This study was funded by Genentech, Inc. Support for third-party writing assistance for this manuscript was provided by Genentech, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DL, CL, PA, S-CC, AQ, JYJ, and SG are employees of Genentech and own stock in F. Hoffmann-La Roche; MP and AS are employees of and own stock in F. Hoffmann-La Roche; MR, DP, and JF are salaried employees of Metrum Research Group, which was contracted by Genentech, Inc.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, D., Li, C., Riggs, M. et al. Pharmacokinetics of trastuzumab emtansine (T-DM1) as a single agent or in combination with pertuzumab in HER2-positive breast cancer patients with recurrent or locally advanced metastatic breast cancer. Cancer Chemother Pharmacol 84, 175–185 (2019). https://doi.org/10.1007/s00280-019-03852-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03852-z