Abstract
Purpose
To determine the recommended dose (RD) of gemcitabine (GEM) plus S-1 (GS) in curatively resected biliary tract cancer (BTC) patients without major hepatectomy.
Methods
A standard 3 + 3 dose-escalation design was used with planned dose levels (mg/m2) of GEM (administered intravenously on days 1 and 8) and S-1 (administered orally twice daily on days 1–14, with a 1-week rest, every 3 weeks for up to 24 weeks) of 1000/80 (Level 2), 1000/65 (Level 1), 800/65 (Level − 1), and 800/50 (Level − 2).
Results
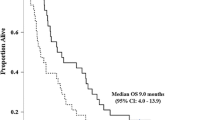
Thirty-one patients (17 men and 14 women; median age, 70 years) were enrolled. Level 1 was chosen as the starting dose. Three of seven patients developed dose-limiting toxicities at Level 1 and the dose was de-escalated to Level − 1. Five of 12 patients developed Grade 4 neutropenia at Level − 1 and the dose was de-escalated to Level − 2. One patient developed Grade 4 neutropenia at Level − 2. Another patient was unable to receive the day 8 dose due to Grade 3 neutropenia at Level − 2. Level − 1 was confirmed as the maximum tolerated dose and Level − 2 the RD for this regimen. The 1- and 2-year recurrence-free survival rates were 77.0 and 54.0%, respectively. The recurrence-free survival rate of patients in the GS completion group was significantly higher than that of the GS discontinuation group.
Conclusions
Level − 2 was confirmed as the RD (GEM 800 mg/m2 and S-1 50 mg/m2) for GS adjuvant chemotherapy in curatively resected BTC patients without major hepatectomy.



Similar content being viewed by others
References
MiyakawaS, IshiharaS, HoriguchiA et al (2009) Biliary tract cancer treatment: 5584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg16:1–7
Labour and Welfare Ministry of Health (2010) Handbook of health and welfare statistics. http://www.mhlw.go.jp/english/database/db-hh/
American Cancer Society (2010) Cancer facts figs. http://www.cancer.org/Research/CancerFactsFigures/
NathanH, PawlikTM, WolfgangCL et al (2007) Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg11:1488–1496 (discussion 14967)
MurakamiY, UemuraK, SudoT et al (2013) Perineural invasion in extrahepatic cholangiocarcinoma: prognostic impact and treatment. J Gastrointest Surg17:1429–1439
DeOliveiraML, CunninghamSC, CameronJL et al (2007) Cholangiocarcinoma: 31-year experience with 564 patients at a single institution. Ann Surg245:755–762
NaginoM, EbataT, YokoyamaY et al (2013) Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg258:129–140
PenzM, KornekGV, RadererM et al (2001) Phase II trial of 2-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol12:183–186
OkusakaT, IshiiH, FunakoshiA et al (2006) Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol57:647–653
UenoH, OkusakaT, IkedaM et al (2004) Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer91:1769–1774
FuruseJ, OkusakaT, BokuN et al (2008) S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol62:849–855
ParkI, LeeJL, RyuMH et al (2009) Efficacy and safety of S-1 monotherapy in patients with advanced biliary tract adenocarcinoma: retrospective analysis of 162 patients. Oncology76:126–132
KanaiM, YoshimuraK, TsumuraT et al (2011) A multi-institution phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother Pharmacol67:1429–1434
SasakiT, IsayamaH, NakaiY et al (2010) Multicenter, phase II study of gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol65:1101–1107
MorizaneC, OkusakaT, MizusawaJ et al (2013) Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci104:1211–1216
YamanakaK, HatanoE, KanaiM et al (2014) A single-center analysis of the survival benefits of adjuvant gemcitabine chemotherapy for biliary tract cancer. Int J Clin Oncol19:485–489
TakaharaT, NittaH, HasegawaY et al (2012) A phase I study for adjuvant chemotherapy of gemcitabine plus S-1 in curatively resected patients with biliary tract cancer: adjusting the dose of adjuvant chemotherapy according to the surgical procedures. Cancer Chemother Pharmacol69:1127–1133
TakadaT, AmanoH, YasudaH et al (2002) Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer95:1685–1695
KobayashiS, NaganoH, SakaiD et al (2014) Phase I study of adjuvant gemcitabine or S-1 in patients with biliary tract cancers undergoing major hepatectomy: KHBO1003 study. Cancer Chemother Pharmacol74:699–709
MurakamiY, UemuraK, SudoT et al (2009) Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg250:950–956
MurakamiY, UemuraK, SudoT et al (2012) Adjuvant chemotherapy with gemcitabine and S-1 after surgical resection for advanced biliary carcinoma: outcomes and prognostic factors. J Hepatobiliary Pancreat Sci19:306–313
ToyodaM, AjikiT, FujiwaraY et al (2014) Phase I study of adjuvant chemotherapy with gemcitabine plus cisplatin in patients with biliary tract cancer undergoing curative resection without hepatectomy (KHB01004). Cancer Chemother Pharmacol73:1295–1301
KainumaO, MiuraF, FurukawaD et al (2015) Feasibility and efficacy of gemcitabine plus cisplatin combination therapy after curative resection for biliary tract cancer. J Hepatobiliary Pancreat Sci22:789–94
Acknowledgements
Acknowledgments we thank all the people related to this study, patients, caregivers, physicians, medical workers, data manager, Ms. Masami Kashibou for data management. This work was Independent Research and Development.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tatsuya Ioka received research funding from Taiho Pharmaceutical Co. All remaining authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studieswith animals performed by any of the authors.
Ethical approval
All procedures performed in studies involvinghuman participants were in accordance with the ethical standards ofthe institutional research committee and with the 1964 Declaration ofHelsinki and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from allstudy participants.
Rights and permissions
About this article
Cite this article
Yanagimoto, H., Toyokawa, H., Sakai, D. et al. A phase I study for adjuvant chemotherapy of gemcitabine plus S-1 in patients with biliary tract cancer undergoing curative resection without major hepatectomy (KHBO1202). Cancer Chemother Pharmacol 81, 461–468 (2018). https://doi.org/10.1007/s00280-017-3513-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3513-4