Abstract
Purpose
The β-nitrostyrene family has been previously reported to possess anticancer property. However, the biological effects of β-nitrostyrenes on ovarian cancer and the underlying mechanisms involved remain unclear. In the present study, we synthesized a β-nitrostyrene derivative, CYT-Rx20 3′-hydroxy-4′-methoxy-β-methyl-β-nitrostyrene), and investigated its anticancer effects and the putative pathways of action in ovarian cancer.
Methods
The effects of CYT-Rx20 were analyzed using cell viability assay, reactive oxygen species (ROS) generation assay, FACS analysis, annexin V staining, immunostaining, comet assay, immunoblotting, soft agar assay, migration assay, nude mice xenograft study and immunohistochemistry.
Results
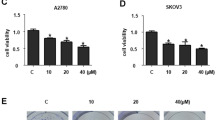
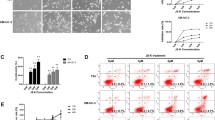
CYT-Rx20 induced cytotoxicity in ovarian cancer cells by promoting cell apoptosis via ROS generation and DNA damage. CYT-Rx20-induced cell apoptosis, ROS generation and DNA damage were reversed by thiol antioxidants. In addition, CYT-Rx20 inhibited ovarian cancer cell migration by regulating the expression of epithelial to mesenchymal transition (EMT) markers. In nude mice, CYT-Rx20 inhibited ovarian tumor growth accompanied by increased expression of DNA damage marker γH2AX and decreased expression of EMT marker Vimentin.
Conclusions
CYT-Rx20 inhibits ovarian cancer cells in vitro and in vivo, and has the potential to be further developed into an anti-ovarian cancer drug clinically.





Similar content being viewed by others

References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics. CA Cancer J Clin 63(1):11–30. doi:10.3322/caac.21166
Sherman-Baust CA, Becker KG, Wood Iii WH, Zhang Y, Morin PJ (2011) Gene expression and pathway analysis of ovarian cancer cells selected for resistance to cisplatin, paclitaxel, or doxorubicin. J Ovarian Res 4(1):21. doi:10.1186/1757-2215-4-21
Romero I, Bast RC Jr (2012) Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology 153(4):1593–1602. doi:10.1210/en.2011-2123
Park J, Pei D (2004) Trans-beta-nitrostyrene derivatives as slow-binding inhibitors of protein tyrosine phosphatases. Biochemistry 43(47):15014–15021. doi:10.1021/bi0486233
Chen IH, Chang FR, Wu YC, Kung PH, Wu CC (2015) 3,4-Methylenedioxy-beta-nitrostyrene inhibits adhesion and migration of human triple-negative breast cancer cells by suppressing beta1 integrin function and surface protein disulfide isomerase. Biochimie 110:81–92. doi:10.1016/j.biochi.2015.01.006
Rahmani-Nezhad S, Safavi M, Pordeli M, Ardestani SK, Khosravani L, Pourshojaei Y, Mahdavi M, Emami S, Foroumadi A, Shafiee A (2014) Synthesis, in vitro cytotoxicity and apoptosis inducing study of 2-aryl-3-nitro-2H-chromene derivatives as potent anti-breast cancer agents. Eur J Med Chem 86:562–569. doi:10.1016/j.ejmech.2014.09.017
Hsieh PW, Chang YT, Chuang WY, Shih HC, Chiang SZ, Wu CC (2010) The synthesis and biologic evaluation of anti-platelet and cytotoxic beta-nitrostyrenes. Bioorg Med Chem 18(21):7621–7627. doi:10.1016/j.bmc.2010.08.039
He Y, Varadarajan S, Munoz-Planillo R, Burberry A, Nakamura Y, Nunez G (2014) 3,4-Methylenedioxy-beta-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem 289(2):1142–1150. doi:10.1074/jbc.M113.515080
Carter KC, Finnon YS, Daeid NN, Robson DC, Waddell R (2002) The effect of nitrostyrene on cell proliferation and macrophage immune responses. Immunopharmacol Immunotoxicol 24(2):187–197. doi:10.1081/iph-120003749
Zeng Z, Sun Z, Huang M, Zhang W, Liu J, Chen L, Chen F, Zhou Y, Lin J, Huang F, Xu L, Zhuang Z, Guo S, Alitongbieke G, Xie G, Xu Y, Lin B, Cao X, Su Y, Zhang XK, Zhou H (2015) Nitrostyrene derivatives act as RXRalpha ligands to inhibit TNFalpha activation of NF-kappaB. Cancer Res 75(10):2049–2060. doi:10.1158/0008-5472.can-14-2435
Hung AC, Tsai CH, Hou MF, Chang WL, Wang CH, Lee YC, Ko A, Hu SC, Chang FR, Hsieh PW, Yuan SS (2016) The synthetic beta-nitrostyrene derivative CYT-Rx20 induces breast cancer cell death and autophagy via ROS-mediated MEK/ERK pathway. Cancer Lett 371(2):251–261. doi:10.1016/j.canlet.2015.11.035
Wang WY, Hsieh PW, Wu YC, Wu CC (2007) Synthesis and pharmacological evaluation of novel beta-nitrostyrene derivatives as tyrosine kinase inhibitors with potent antiplatelet activity. Biochem Pharmacol 74(4):601–611. doi:10.1016/j.bcp.2007.06.001
Chen HM, Wu YC, Chia YC, Chang FR, Hsu HK, Hsieh YC, Chen CC, Yuan SS (2009) Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett 286(2):161–171. doi:10.1016/j.canlet.2009.05.040
Yuan SS, Hou MF, Hsieh YC, Huang CY, Lee YC, Chen YJ, Lo S (2012) Role of MRE11 in cell proliferation, tumor invasion, and DNA repair in breast cancer. J Natl Cancer Inst 104(19):1485–1502. doi:10.1093/jnci/djs355
Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC (1996) Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol 148(5):1567–1576
Alfadda AA, Sallam RM (2012) Reactive oxygen species in health and disease. J Biomed Biotechnol 2012:936486. doi:10.1155/2012/936486
Costa A, Scholer-Dahirel A, Mechta-Grigoriou F (2014) The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol 25:23–32. doi:10.1016/j.semcancer.2013.12.007
Reczek CR, Chandel NS (2015) ROS-dependent signal transduction. Curr Opin Cell Biol 33:8–13. doi:10.1016/j.ceb.2014.09.010
Kuo LJ, Yang LX (2008) Gamma-H2AX-a novel biomarker for DNA double-strand breaks. In vivo 22(3):305–309
Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta 12:3481–3498. doi:10.1016/j.bbamcr.2013.06.026 (pii:S0167-4889(13)00249-8)
Scheel C, Weinberg RA (2012) Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol 22(5–6):396–403. doi:10.1016/j.semcancer.2012.04.001
Tsai JH, Yang J (2013) Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 27(20):2192–2206. doi:10.1101/gad.225334.113
Dickinson BC, Chang CJ (2011) Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol 7(8):504–511. doi:10.1038/nchembio.607
Pan ST, Qin Y, Zhou ZW, He ZX, Zhang X, Yang T, Yang YX, Wang D, Qiu JX, Zhou SF (2015) Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK- and PI3 K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells. Drug Des Devel Ther 9:1601–1626. doi:10.2147/dddt.s76057
Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW, Peehl DM, Knox SJ (2002) Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ 9(3):252–263. doi:10.1038/sj.cdd.4400959
Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ (2005) Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal 7(1–2):42–59. doi:10.1089/ars.2005.7.42
Burney S, Niles JC, Dedon PC, Tannenbaum SR (1999) DNA damage in deoxynucleosides and oligonucleotides treated with peroxynitrite. Chem Res Toxicol 12(6):513–520. doi:10.1021/tx980254m
Kang MA, So EY, Simons AL, Spitz DR, Ouchi T (2012) DNA damage induces reactive oxygen species generation through the H2AX-Nox1/Rac1 pathway. Cell death Dis 3:e249. doi:10.1038/cddis.2011.134
Bekker-Jensen S, Mailand N (2010) Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair 9(12):1219–1228. doi:10.1016/j.dnarep.2010.09.010
Olive PL, Banath JP (2006) The comet assay: a method to measure DNA damage in individual cells. Nat Protoc 1(1):23–29. doi:10.1038/nprot.2006.5
Yang X, Zheng F, Xing H, Gao Q, Wei W, Lu Y, Wang S, Zhou J, Hu W, Ma D (2004) Resistance to chemotherapy-induced apoptosis via decreased caspase-3 activity and overexpression of antiapoptotic proteins in ovarian cancer. J Cancer Res Clin Oncol 130(7):423–428. doi:10.1007/s00432-004-0556-9
Nicholson DW, Thornberry NA (1997) Caspases: killer proteases. Trends Biochem Sci 22(8):299–306
Voulgari A, Pintzas A (2009) Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 1796(2):75–90. doi:10.1016/j.bbcan.2009.03.002
Haslehurst AM, Koti M, Dharsee M, Nuin P, Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, Davey S, Squire J, Park PC, Feilotter H (2012) EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer 12:91. doi:10.1186/1471-2407-12-91
Acknowledgements
This study was supported by Grants from the Ministry of Health and Welfare (MOHW105-TDU-B-212-134007 and MOHW105-TDU-B-212-112016, Health and welfare surcharge of tobacco products) of Taiwan and National Health Research Institutes (NHRI-EX104-10212BI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yen-Yun Wang, Yuk-Kwan Chen, Stephen Chu-Sung Hu, Ya-Ling Hsu, Chun-Hao Tsai, Tsung-Chen Chi, Wan-Ling Huang, Pei-Wen Hsieh, and Shyng-Shiou F. Yuan have declared no conflict of interest.
Ethical approval
The animal studies were approved by the Institutional Animal Care and Use Committee (IACUC No. 102009) of Kaohsiung Medical University, Taiwan. Animal experiments were approved by the Laboratory Animal Ethics Committee of Kaohsiung Medical University.
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2017_3330_MOESM1_ESM.pdf
Supplementary Fig. 1 Chemical structures of the β-nitrostyrene derivatives CYT-Rx20, CYT-Rx21, CYT-Rx22, CYT-Rx44, CYT-Rx45, CYT-Rx46, and CYT-Rx47 (PDF 43 kb)
280_2017_3330_MOESM2_ESM.pdf
Supplementary Fig. 2a Cells were treated with CYT-Rx20 for 1 h and ROS level was determined by staining with H2DCFDA fluorescent dye and analysis by flow cytometry. b Cells were treated with various concentrations of CYT-Rx20 (2 μg/ml) for 24 h prior to the determination of DNA damage by neutral comet assay. c Effect of CYT-Rx20 on induction of γ-H2AX focus formation in ovarian cancer cells. After treatment with the indicated concentrations of CYT-Rx20 (2 μg/ml) for 24 h, cells were fixed and incubated with antibodies against γ-H2AX, followed by secondary antibodies conjugated with the fluorochrome FITC. Nuclei were stained with DAPI. After immunostaining, cells were viewed by fluorescence microscope (original magnification × 1000) (PDF 218 kb)
280_2017_3330_MOESM3_ESM.pdf
Supplementary Fig. 3 MDAH 2774, PA-1, and SKOV3 cells were treated with various concentrations of CYT-Rx20 for 24 h and then cell viability was assessed by the XTT colorimetric assay (PDF 31 kb)
280_2017_3330_MOESM4_ESM.pdf
Supplementary Fig. 4 a The body weights of the nude mice included in this study were measured every week. b Hematoxylin and eosin staining of the tissues from mice organs. The representative photographs were shown with ×200 magnification. Bar represents 200 μm (PDF 466 kb)
Rights and permissions
About this article
Cite this article
Wang, YY., Chen, YK., Hu, S.CS. et al. CYT-Rx20 inhibits ovarian cancer cells in vitro and in vivo through oxidative stress-induced DNA damage and cell apoptosis. Cancer Chemother Pharmacol 79, 1129–1140 (2017). https://doi.org/10.1007/s00280-017-3330-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3330-9