Abstract
Background
Autophagy is a survival mechanism that allows recycling of cellular breakdown products, particularly in stressed cells. Here we evaluate the hypotheses that up-regulation of autophagy is a common mechanism of resistance to chemotherapy, and that drug resistance can be reversed by inhibiting autophagy with a proton pump inhibitor.
Methods
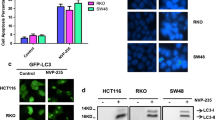
We exposed human PC3, LNCaP and MCF7 cells to seven clinically-used chemotherapy drugs ± pantoprazole, examined the up-regulation of autophagy and the effect on cellular proliferation by Western Blots, MTS assay and colony-forming assay. The distribution of drug effects and of autophagy was quantified in LNCaP tumor sections in relation to blood vessels and hypoxia by immunohistochemistry using γH2AX, cleaved caspase-3 and p62.
Results
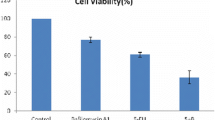
All anticancer drugs led to up-regulation of autophagy in cultured tumor cells. Pantoprazole inhibited the induction of autophagy in a time- and dose-dependent manner, and sensitized cancer cells to the seven anti-cancer drugs. Treatment of LNCaP xenografts with paclitaxel induced both DNA damage and autophagy; autophagy was inhibited and markers of toxicity were increased by pantoprazole.
Conclusions
Induction of autophagy is a general mechanism associated with resistance to anticancer drugs and that its inhibition is a promising therapeutic strategy to enhance the effects of chemotherapy and improve clinical outcomes.





Similar content being viewed by others
Change history
19 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00280-023-04636-2
References
Tredan O et al (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99(19):1441–1454
Fukumura D, Jain RK (2007) Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res 74(2–3):72–84
Davis AJ, Tannock JF (2000) Repopulation of tumour cells between cycles of chemotherapy: a neglected factor. Lancet Oncol 1:86–93
Rouschop KM et al (2010) The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest 120(1):127–141
Mizushima N, Ohsumi Y, Yoshimori T (2002) Autophagosome formation in mammalian cells. Cell Struct Funct 27(6):421–429
Tsukada M, Ohsumi Y (1993) Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett 333(1–2):169–174
Alva AS, Gultekin SH, Baehrecke EH (2004) Autophagy in human tumors: cell survival or death? Cell Death Differ 11(9):1046–1048
Liang XH et al (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402(6762):672–676
Boya P et al (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25(3):1025–1040
Fujii S et al (2008) Autophagy is activated in pancreatic cancer cells and correlates with poor patient outcome. Cancer Sci 99(9):1813–1819
Yang ZJ et al (2011) The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther 10(9):1533–1541
Amaravadi RK et al (2011) Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res 17(4):654–666
Solomon VR, Lee H (2009) Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol 625(1–3):220–233
Marino ML et al (2010) Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis 1:e87
Luciani F et al (2004) Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst 96(22):1702–1713
Patel KJ et al (2013) Use of the proton pump inhibitor pantoprazole to modify the distribution and activity of doxorubicin: a potential strategy to improve the therapy of solid tumors. Clin Cancer Res 19(24):6766–6776
Lindner K et al (2014) Proton pump inhibitors (PPIs) impact on tumour cell survival, metastatic potential and chemotherapy resistance, and affect expression of resistance-relevant miRNAs in esophageal cancer. J Exp Clin Cancer Res 33(1):73
Tan Q et al., Effect of pantoprazole to enhance activity of docetaxel against human tumour xenografts by inhibiting autophagy. Br J Cancer, 2015
Kimura S et al (2009) Monitoring autophagy in mammalian cultured cells through the dynamics of LC3. Methods Enzymol 452:1–12
Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36(12):2503–2518
Klionsky DJ et al (2008) Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy 4(7):849–850
Bjorkoy G et al (2009) Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol 452:181–197
Kimura S, Noda T, Yoshimori T (2007) Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3(5):452–460
Fung AS, Jonkman J, Tannock IF (2012) Quantitative immunohistochemistry for evaluating the distribution of Ki67 and other biomarkers in tumor sections and use of the method to study repopulation in xenografts after treatment with paclitaxel. Neoplasia 14(4):324–334
Hu YL et al (2012) Tumor cell autophagy as an adaptive response mediating resistance to treatments such as antiangiogenic therapy. Cancer Res 72(17):4294–4299
Zou Z et al (2012) Aurora kinase A inhibition-induced autophagy triggers drug resistance in breast cancer cells. Autophagy 8(12):1798–1810
Amaravadi RK (2008) Autophagy-induced tumor dormancy in ovarian cancer. J Clin Invest 118(12):3837–3840
Yoshioka A et al (2008) LC3, an autophagosome marker, is highly expressed in gastrointestinal cancers. Int J Oncol 33(3):461–468
Ziparo E et al (2013) Autophagy in prostate cancer and androgen suppression therapy. Int J Mol Sci 14(6):12090–12106
Maycotte P, Thorburn A (2014) Targeting autophagy in breast cancer. World J Clin Oncol 5(3):224–240
Liu F et al (2013) Effect of autophagy inhibition on chemotherapy-induced apoptosis in A549 lung cancer cells. Oncol Lett 5(4):1261–1265
Kanzawa T et al (2004) Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ 11(4):448–457
Han W et al (2011) Autophagy inhibition enhances daunorubicin-induced apoptosis in K562 cells. PLoS One 6(12):e28491
de Medina P, Silvente-Poirot S, Poirot M (2009) Tamoxifen and AEBS ligands induced apoptosis and autophagy in breast cancer cells through the stimulation of sterol accumulation. Autophagy 5(7):1066–1067
Dragowska WH et al (2013) Induction of autophagy is an early response to gefitinib and a potential therapeutic target in breast cancer. PLoS One 8(10):e76503
Cruickshanks N et al (2015) Differential regulation of autophagy and cell viability by ceramide species. Cancer Biol Ther 16(5):733–742
Martin AP et al (2009) Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther 8(21):2084–2096
Codogno P, Meijer AJ (2005) Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 12(Suppl 2):1509–1518
Gupta A et al (2010) Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST). Proc Natl Acad Sci USA 107(32):14333–14338
Han W et al (2011) EGFR tyrosine kinase inhibitors activate autophagy as a cytoprotective response in human lung cancer cells. PLoS One 6(6):e18691
Ma XH et al (2011) Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin Cancer Res 17(10):3478–3489
Carew JS et al (2007) Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood 110(1):313–322
Hu C et al (2008) The efficacy and selectivity of tumor cell killing by Akt inhibitors are substantially increased by chloroquine. Bioorg Med Chem 16(17):7888–7893
Rosenfeld MR et al (2014) A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy 10(8):1359–1368
De Milito A et al (2007) Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res 67(11):5408–5417
Wang QJ et al (2006) Induction of autophagy in axonal dystrophy and degeneration. J Neurosci 26(31):8057–8068
Brana I et al (2014) A phase I trial of pantoprazole in combination with doxorubicin in patients with advanced solid tumors: evaluation of pharmacokinetics of both drugs and tissue penetration of doxorubicin. Invest New Drugs 32(6):1269–1277
Acknowledgements
We thank all members of the Pathology Research Program (PRP), and the Advanced Optical Microscopy Facility (AOMF). Special thanks to Dr. Richard Hill for his guidance and helpful advice. Supported by grant KG100252 from the Komen Foundation, a grant from the Canadian Institutes of Health Research and a grant from Prostate Cancer Canada Rising Star award.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that she/he has no conflict of interest.
Ethical approval
Animal experiments described in this paper were carried out using Animal Use Protocol (AUP1232.15, 09/05/14) approved by Princess Margaret Cancer Center, University Health Network (UHN) Animal Care Committee under the guidelines of the Canadian Council on Animal Care.
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2017_3298_MOESM1_ESM.tif
A. Quantitative analysis of relative LC3-II and p62 levels in MCF7, LNCaP and PC3 cells. B. Quantification of RFP+ and GFP+ punctae /cell in MCF7 and PC3 cell lines, ratio between pantoprazole and control, combination treatment and chemotherapy drug alone. Data are means ± SEM (N=3) (TIF 96 KB)
280_2017_3298_MOESM2_ESM.tif
A. Effect of pantoprazole on autophagy. LNCaP cells were treated with bafilomycin A1 (BAF:100nM 4hr), pantoprazole (PTP: 100µM for 26hr), doxorubicin (DOX: 50nM), docetaxel (DOCE: 1nM), gemcitabine (GEM: 50nM), melphalan (MEL: 1µM), methotrexate (MTX: 10nM), mitoxantrone (MIT: 50nM), paclitaxel (PAC: 1nM) alone (for 24hr) or pantoprazole 2 hours prior to chemotherapy drugs (pantoprazole 26 hr commencing 2h prior to chemotherapy drugs). The protein levels of LC3-II and p62 were assayed by western blots (N=3). B. Quantitative analysis of LC3-II and p62 levels in LNCaP cells. The relative density of the LC3-II band is indicated. C. The relative density of the p62 band is indicated. The relative levels of LC3-II and p62 were normalized to β-actin and the value of control was set as 1.0. * p<0.05 chemotherapy drugs treatment alone vs respective control; ** p<0.01 pre-treatment with PTP vs chemotherapy drug treatment alone. Error bars represent SEM for 3 independent experiments (TIF 112 KB)
280_2017_3298_MOESM3_ESM.tif
A. Effect of pantoprazole on autophagy. PC3 cells were treated with bafilomycin A1 100nM (4hr), pantoprazole (100µM for 26hr), doxorubicin (DOX: 50nM), docetaxel (DOCE: 1nM), gemcitabine (GEM: 50nM), melphalan (MEL: 1µM), methotrexate (MTX: 10nM), mitoxantrone (MIT: 50nM), paclitaxel (PAC: 1nM) alone (for 24hr) or pantoprazole 2 hours prior to chemotherapy drugs (pantoprazole 26 hr commencing 2h prior to chemotherapy drugs). The protein levels of LC3-II and p62 were assayed by western blot (N=3). B. Quantitative analysis of LC3-II and p62 levels in PC3 cells: The relative density of the LC3-II band is indicated. C. The relative density of the p62 band is indicated. The relative levels of LC3-II and p62 were normalized to β-actin and the value of control was set as 1.0. * p<0.05 chemotherapy drugs treatment alone vs respective control; ** p<0.01 pre-treatment with PTP vs chemotherapy drug treatment alone). Error bars represent SEM for 3 independent experiments (TIF 126 KB)
280_2017_3298_MOESM4_ESM.tif
LNCaP tumor xenografts treated with paclitaxel, pantoprazole, pantoprazole 2 hours prior to paclitaxel, or untreated controls. Figures represent percent positive pixels of cleaved caspase-3 (A) and Ki67 (C) as a function of distance from the nearest blood vessel and of cleaved caspase-3 (B) and Ki67 (D) as a function of distance from the nearest hypoxic region. Error bars around curves represent SEM for 5–6 mice per group. Differences between combination group and paclitaxel group (p < 0.01), and these two groups compare to control curves in panels B-D are statistically significantly (p < 0.05) (TIF 78 KB)
Rights and permissions
About this article
Cite this article
Tan, Q., Joshua, A.M., Wang, M. et al. Up-regulation of autophagy is a mechanism of resistance to chemotherapy and can be inhibited by pantoprazole to increase drug sensitivity. Cancer Chemother Pharmacol 79, 959–969 (2017). https://doi.org/10.1007/s00280-017-3298-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3298-5