Abstract
Purpose
Trametinib is a reversible, selective inhibitor of the mitogen-activated extracellular signal-regulated kinase 1 (MEK1) and 2 (MEK2). Cardiotoxicity (congestive heart failure, decreased heart rate, left ventricular dysfunction, and hypertension) related to trametinib is an infrequent, but serious, adverse event (AE). Prolongation of the QT interval increases the risk of life-threatening cardiac arrhythmia. Thus, the risk of trametinib inducing QT prolongation at putative supratherapeutic exposure was evaluated.
Methods
Eligible patients with solid tumours received placebo on day 1, once-daily trametinib 2-mg doses on days 2–14, and a single trametinib 3-mg dose on day 15 to achieve supratherapeutic dosing for QTc measurement. Electrocardiogram was assessed by 12-lead ambulatory 24-h Holter monitoring pre-dose, and on day 1 and day 15. Pharmacokinetic (PK) and pharmacodynamics (PD) parameters were measured.
Results
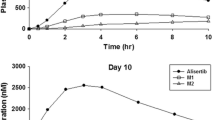
Thirty-two of 35 patients completed the study. There was no effect of trametinib when compared with time-matched placebo on the change from baseline in QTcF, QTcB, or QTcI interval. Mean AUC0–24 and C max following trametinib 2-mg repeat doses were 364 ng.h/mL and 22.9 ng/mL, respectively; the corresponding values for the 3-mg dose were 454 ng.h/mL and 29.2 ng/mL. Median T max was approximately 2 h for both doses. Statistical analysis and PK/PD modelling showed no significant relationship between QTcF interval and trametinib plasma concentrations. AEs were consistent with those reported previously. No electrocardiogram abnormalities were reported as AEs.
Conclusions
The results of this study suggest trametinib has no significant effect on QT prolongation at supratherapeutic exposure.

Similar content being viewed by others
References
Montagut C, Settleman J (2009) Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett 283:125–134
Dhillon AS, Hagan S, Rath O, Kolch W (2007) MAP kinase signalling pathways in cancer. Oncogene 26:3279–3290
Burotto M, Chiou VL, Lee JM, Kohn EC (2014) The MAPK pathway across different malignancies: a new perspective. Cancer 120:3446–3456
Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, Dummer R, Trefzer U, Larkin JM, Utikal J, Dreno B, Nyakas M, Middleton MR, Becker JC, Casey M, Sherman LJ, Wu FS, Ouellet D, Martin AM, Patel K, Schadendorf D, METRIC Study Group (2012) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367:107–114
Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, Haanen JBAG, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Casey M, Ouellet D, Martin A-M, Le N, Patel K, Flaherty K (2014) Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877–1888
Mekinist (trametinib) [package insert] (2015) Research triangle park, NC: GlaxoSmithKline
Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, Burris HA 3rd, Falchook G, Algazi A, Lewis K, Long GV, Puzanov I, Lebowitz P, Singh A, Little S, Sun P, Allred A, Ouellet D, Kim KB, Patel K, Weber J (2012) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367:1694–1703
Nachimuthu S, Assar MD, Schussler JM (2012) Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf 3:241–253
Bagchi C, Sinha DP, Tripathi SK (2010) A suspected case of carbimazole-associated torsades de pointes. Indian J Pharmacol 42:53–54
Ahmed N, Riaz K, Rai R, Osman M, Wase A (2006) Congenital long QT syndrome. Rev Cardiovasc Med 7:160–165
Barcelos AM, Teixeira MA, Maia Mda C, Camanho LE, Assumpcao OQ (2009) Postpartum torsades de pointes and long QT syndrome. Arq Bras Cardiol 93(e58–9):e46–e47
Meyer JS, Mehdirad A, Salem BI, Kulikowska A, Kulikowski P (2003) Sudden arrhythmia death syndrome: importance of the long QT syndrome. Am Fam Physician 68:483–488
Woodcock J, US Food and Drug Administration (1997) Docket No 96 N-0512. Hoechst Marion Roussel Inc, and Baker Norton Pharmaceuticals, Inc.; Terfenadine; proposal to withdraw approval of two new drug applications and one abbreviated new drug application; opportunity for a hearing. Fed Reg 62:1889–1892
Wysowski DK, Corken A, Gallo-Torres H, Talarico L, Rodriguez EM (2001) Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol 96:1698–1703
Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, DeMarini DJ, Cox DS, Xu Y, Morris SR, Peddareddigari VG, Le NT, Hart L, Bendell JC, Eckhardt G, Kurzrock R, Flaherty K, Burris HA 3rd, Messersmith WA (2012) Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol 13:773–781
Shah RR, Morganroth J (2015) Update on cardiovascular safety of tyrosine kinase inhibitors: with a special focus on QT interval, left ventricular dysfunction and overall risk/benefit. Drug Saf 38:693–710
Becker TK, Yeung SJ (2010) Drug-induced QT interval prolongation in cancer patients. Oncol Rev 4:223–232
US Department of Health and Human Services, Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) (2013) Application number: 204114Orig1s000; pharmacology review(s)
US Department of Health and Human Services, Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) (2013). Application number: 204114Orig1s000; clinical pharmacology and biopharmaceutics review(s)
Acknowledgments
We thank the patients who participated in this study and all of the personnel involved in patient care and data collection. The authors wish to acknowledge the following individuals for their contribution and critical review during development of this manuscript: Bijoyesh Mookerjee, Anny Chen, and Stanley Carson. Financial support for medical editorial assistance was provided by Novartis Oncology. We also thank Heather Edens, Ph.D. (ArticulateScience, LLC, Hamilton, NJ), for her medical editorial assistance with this manuscript.
Funding
This study was sponsored by GlaxoSmithKline; however, as of 2 March 2015, trametinib became an asset of Novartis AG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TLW reports no competing interests; KPP, MB, and SS received research funding from GlaxoSmithKline for the submitted work; AP received research funding to their institution from GlaxoSmithKline; AT and DR received research funding to their institution from Merck. JWB, AS, DSC, BRP, YZ, MH, and DS were employees of GlaxoSmithKline in the previous 3 years (at the time this study was conducted); DSC owned stock in GlaxoSmithKline in the previous 3 years; SS participated in an advisory board for GlaxoSmithKline. There are no other relationships or activities that could appear to have influenced the submitted work.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from each patient prior to performance of any study-specific procedures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2016_3090_MOESM1_ESM.pdf
Supplementary material 1 Supplement Fig. 1 Visual predictive check goodness-of-fit plots by dose group. A total of 500 simulations were performed with the final pharmacokinetic/pharmacokinetic parameters for (A) placebo, (B) trametinib 2 mg, and (C) trametinib 3 mg to compare the distribution of simulated QTcF intervals with that of the observed data. The majority of observed data fell within the range between the 5th and 95th percentiles of the simulated values (grey zones), indicating that the final model sufficiently describes the observed QTcF data. QTcF, QT interval corrected for heart rate by the Fridericia formula (PDF 918 kb)
Rights and permissions
About this article
Cite this article
Patnaik, A., Tolcher, A., Papadopoulos, K.P. et al. Phase 1 study to evaluate the effect of the MEK inhibitor trametinib on cardiac repolarization in patients with solid tumours. Cancer Chemother Pharmacol 78, 491–500 (2016). https://doi.org/10.1007/s00280-016-3090-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3090-y