Abstract
Purpose
To confirm the safety and tolerability, evaluate the pharmacokinetics (PK), and investigate the antitumor activity of abemaciclib in Japanese patients with advanced cancer.
Methods
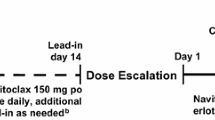
We conducted a non-randomized, single-arm, open-label, dose-escalation phase 1 study of abemaciclib administered orally every 12 h (Q12H) on a 28-day cycle at doses of 100 mg (Cohort 1, n = 3), 150 mg (Cohort 2, n = 3), or 200 mg [Cohort 3, n = 6, maximum tolerated dose (MTD)]. Dose escalation was based on the frequency of dose-limiting toxicity (DLT). MTD, as established in the previous phase 1 study in non-Japanese patients, was the highest dose level at which <33 % of patients experienced DLT.
Results
Eleven of the 12 patients who received treatment with abemaciclib discontinued: 10 patients due to progressive disease, and 1 due to a DLT (Cohort 3, grade 2 nausea). Diarrhea, the most common treatment-emergent adverse event (AE), was managed supportively and did not require study treatment discontinuation. There were no drug-related serious AEs and no patients with corrected QT (QTc) > 480 ms or QTc change of >60 ms from baseline. The abemaciclib PK profile was characterized by slow absorption and high PK variability after single or repeated doses. Two patients, one with breast cancer and one with neuroendocrine tumor, experienced >30 % decrease in tumor size from baseline.
Conclusions
In Japanese patients with advanced cancer, single-agent abemaciclib has an acceptable safety profile and demonstrates antitumor activity at a dose of 200 mg Q12H. These findings support ongoing development of abemaciclib for diverse populations with advanced cancer.



Similar content being viewed by others
References
Lundberg AS, Weinberg RA (1998) Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol 18(2):753–761
Malumbres M (2014) Cyclin-dependent kinases. Genome Biol 15(6):122
Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ et al (2014) Preclinical characterization of the CDK4/6 inhibitor LY2835219: in vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 32(5):825–837
Puyol M, Martin A, Dubus P, Mulero F, Pizcueta P, Khan G, Guerra C, Santamaria D, Barbacid M (2010) A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 18(1):63–73
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35
Vidula N, Rugo HS (2016) Cyclin-dependent kinase 4/6 inhibitors for the treatment of breast cancer: a review of preclinical and clinical data. Clin Breast Cancer 16(1):8–17
Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H et al (2015) Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373(3):209–219
Munster PN, Hamilton EP, Estevez LG, De Boer RH, Mayer IA, Campone M et al (2014) Ph IB study of LEE011 and BYL719 in combination with letrozole in ER+, HER2- breast cancer. J Clin Oncol 32, 2014 (suppl 26; abstr 143). http://meetinglibrary.asco.org/content/127461-144. Accessed 28 Feb 2016
Llombart A, Toi M, Klise SR, Frenzel M, Chan EM, Sledge GW (2014) A phase III study of abemaciclib (LY2835219) combined with fulvestrant in women with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2−) breast cancer (MONARCH 2) Proceedings of the Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium: 2014 Dec 9–13; San Antonio, TX Philadelphia (PA): AACR; Cancer Res 2015;75(9 Suppl):Abstract nr OT1-1-07 Accessed 15 March 2016
Goetz P, Toi M, Klise S, Frenzel M, Bourayou N (2015) MONARCH 3: A randomized phase III study of anastrozole or letrozole plus abemaciclib, a CDK4/6 inhibitor, or placebo in first-line treatment of women with HR, HER2-locoregionally recurrent or metastatic breast cancer (MBC). J Clin Oncol 33(15S, part 1):51; Abstract TPS624
Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, del Prado M, Lallena MJ et al (2011) Abstract B233: identification and characterization of LY2835219: A potent oral inhibitor of the cyclin-dependent kinases 4 and 6 (CDK4/6) with broad in vivo antitumor activity. Mol Cancer Ther 10(11 Supplement):B233
Shapiro G, Rosen LS, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP et al (2013) A first-in-human phase I study of the CDK4/6 inhibitor, LY2835219, for patients with advanced cancer. J Clin Oncol 31(15s):2500
Morschhauser F, Bouabdallah K, Stilgenbauer S, Thieblemont C, Wolf M, de Guibert S et al (2014) Clinical activity of abemaciclib (LY2835219), a cell cycle inhibitor selective for CDK4 and CDK6, in patients with relapsed or refractory mantle cell lymphoma. Blood 124(21):3067
Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L et al (2014) LY2835219, a novel cell cycle inhibitor selective for CDK4/6, in combination with fulvestrant for patients with hormone receptor positive (HR+) metastatic breast cancer. J Clin Oncol 32(15s):534
Tolaney SM, Rosen LS, Beeram M, Goldman JW, Gandhi L, Tolcher AW et al (2015) Abstract P5-19-13: clinical activity of abemaciclib, an oral cell cycle inhibitor, in metastatic breast cancer. Cancer Res 75(9 Supplement):P5-19-13
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Le Tourneau C, Lee JJ, Siu LL (2009) Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 101(10):708–720
Funding support
This study was sponsored by Eli Lilly Japan K.K, manufacturer/licensee of abemaciclib (LY2835219). Medical writing assistance was provided by Mark Snape, MB BS, CMPP and Tania Dickson, PhD of ProScribe—Envision Pharma Group—and was funded by Eli Lilly. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Other contributors/acknowledgments
The authors would like to thank study participants and their families.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kitano, Tanabe, Tamura, and Shimomura have no conflicts of interest to declare. Kondo has received research funding from AstraZeneca, Eli Lilly Japan K.K., and Bayer Yakuhin, Ltd. Yamamoto has received research funding for clinical trials from Chugai Pharmaceutical Co., Ltd, Taiho Pharma, Eisai Co., Ltd, Quintiles, Astellas, Bristol-Myers Squibb, Kyowa-Hakko Kirin, Novartis Pharmaceuticals Japan, Daiichi-Sankyo, Pfizer, and Boehringer Ingelheim. Iwasa has received research funding for clinical trials from Eli Lilly Japan K.K. Fujiwara has served on advisory boards for Novartis Pharmaceuticals Japan and ONO Pharmaceuticals Japan, and received research funding for clinical trials from AstraZeneca, Eli Lilly Japan K.K, Chugai, Eisai Co., Daiichi-Sankyo, and MerckSerono. Ogasawara, Mori, and Asou are current employees of Eli Lilly Japan K.K. Turner and Chan are current employees of and own stock in Eli Lilly and Company.
Role of the sponsor
Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Role of contributors
All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. KO, PKT, HA, and EMC were involved in the study design and data analyses. S Kitano, S Kondo, NY, KT, YT, SI, AS, and YF were investigators in the study and were involved in data collection. KO and JM conducted the statistical analysis.
Rights and permissions
About this article
Cite this article
Fujiwara, Y., Tamura, K., Kondo, S. et al. Phase 1 study of abemaciclib, an inhibitor of CDK 4 and 6, as a single agent for Japanese patients with advanced cancer. Cancer Chemother Pharmacol 78, 281–288 (2016). https://doi.org/10.1007/s00280-016-3085-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-3085-8