Abstract
Purpose
To characterize amatuximab pharmacokinetics (PK) and the relationship of amatuximab exposure with response in patients with unresectable malignant pleural mesothelioma (MPM) receiving amatuximab with pemetrexed and cisplatin.
Methods
A nonlinear mixed effects PK model was built using data from all of the amatuximab studies conducted to date. Patients received amatuximab alone or in combination with chemotherapy. The influence of demographic, laboratory and disease characteristics on PK parameters was assessed. Exposure–response analyses explored relationships between amatuximab exposure and overall survival (OS), progression-free survival (PFS) and safety. Alternative amatuximab dosing regimens were explored with simulations using population PK and parametric survival models.
Results
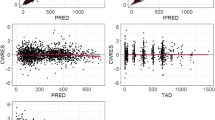
Amatuximab PK was best described by a two-compartment model with parallel linear and nonlinear elimination pathways. Body weight and an antidrug antibodies reaction with the titer >64 affected volume of distribution and clearance, respectively. Exposure–response analyses demonstrated that the amatuximab exposure (C min) showed a significant effect on OS (log-rank test, P = 0.0202). For patients with amatuximab C min above the median (38.2 μg/mL), the median OS was 583 days (90 % CI 418 –NE). For patients with C min ≤ 38.2 μg/mL, the median OS was 375 days (90 % CI 325–486). The amatuximab exposure showed similar significant effect on PFS. Exposure–response analysis for adverse events did not reveal any relationship.
Conclusions
In patients with MPM, higher amatuximab exposure in combination with chemotherapy was shown to be associated with longer OS, supporting evaluation of more frequent dosing in future trials to achieve higher exposure and subsequently longer OS.



Similar content being viewed by others
References
Sugarbaker DJ, Garcia JP, Richards WG et al (1996) Extrapleural pneumonectomy in the multimodality therapy of malignant pleural mesothelioma—Results in 120 consecutive patients. Ann Surg 224:288–294
Vogelzang NJ, Rusthoven JJ, Symanowski J et al (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636–2644
Manzini VD, Brollo A, Franceschi S et al (1993) Prognostic factors of malignant mesothelioma of the pleura. Cancer 72:410–417
Chang K, Pastan I, Willingham MC (1992) Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer 50:373–381
Ordonez NG (2003) Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol 16:192–197
Hassan R, Laszik ZG, Lerner M et al (2005) Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol 124:838–845
Argani P, Iacobuzio-Donahue C, Ryu B et al (2001) Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res 7:3862–3868
Hassan R, Kreitman RJ, Pastan I, Willingham MC (2005) Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol 13:243–247
Miettinen M, Sarlomo-Rikala M (2003) Expression of calretinin, thrombomodulin, keratin 5, and mesothelin in lung carcinomas of different types: an immunohistochemical analysis of 596 tumors in comparison with epithelioid mesotheliomas of the pleura. Am J Surg Pathol 27:150–158
Hassan R, Schweizer C, Lu KF et al (2010) Inhibition of mesothelin-CA125 interaction in patients with mesothelioma by the anti-mesothelin monoclonal antibody MORAb-009: implications for cancer therapy. Lung Cancer 68:455–459
Kelly RJ, Sharon E, Pastan I, Hassan R (2012) Mesothelin-targeted agents in clinical trials and in preclinical development. Mol Cancer Ther 11:517–525
Hassan R, Ebel W, Routhier EL et al (2007) Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun 7:20
Hassan R, Cohen SJ, Phillips M et al (2010) Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 16:6132–6138
Hassan R, Jahan T, Kindler HL et al (2014) Amatuximab, a chimeric monoclonal antibody to mesothelin in combination with pemetrexed and cisplatin in patients with unresectable pleural mesothelioma: results of a multi-center phase II clinical trial. Clin Cancer Res 20:5927–5936
Fujisaka Y, Kurata T, Tanaka K et al (2015) Phase I study of amatuximab, a novel monoclonal antibody to mesothelin, in Japanese patients with advanced solid tumors. Invest New Drugs 33:380–388
Beal SL (2001) Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28:481–504
Dirks NL, Meibohm B (2010) Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49:633–659
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82:17–20
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151
Bergstrand M, Karlsson MO (2009) Handling data below the limit of quantification in mixed effect models. AAPS J 11:371–380
Mould DR, Upton RN (2013) Basic concepts in population modeling, simulation, and model-based drug development—Part 2: introduction to pharmacokinetic modeling methods. Pharmacometrics & Systems Pharmacology, CPT, p e38. doi:10.1038/psp.2013.14
Mould D, Green B (2010) Pharmacokinetics and pharmacodynamics of monoclonal antibodies. Concepts and lessons for drug development. Biodrugs 24:23–39
Acknowledgments
The study was funded by Morphotek/Eisai.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Anubha Gupta is a former employee of Eisai and Ziad Hussein is employed by Eisai. Bruce A. Wallin and Jason Wustner are employed by Morphotek, a subsidiary of Eisai. Julia D. Maltzman is a former employee of Morphotek. Raffit Hassan does not have a potential conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gupta, A., Hussein, Z., Hassan, R. et al. Population pharmacokinetics and exposure–response relationship of amatuximab, an anti-mesothelin monoclonal antibody, in patients with malignant pleural mesothelioma and its application in dose selection. Cancer Chemother Pharmacol 77, 733–743 (2016). https://doi.org/10.1007/s00280-016-2984-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-016-2984-z